J Korean Ophthalmol Soc.
2017 Dec;58(12):1410-1415. 10.3341/jkos.2017.58.12.1410.
A Case of Mucosa-associated Lymphoid Tissue Lymphoma Discovered by Repetitive Intraocular Lens Dislocation
- Affiliations
-
- 1The Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. yoonjs@yuhs.ac
- KMID: 2397856
- DOI: http://doi.org/10.3341/jkos.2017.58.12.1410
Abstract
- PURPOSE
To report a case where bilateral malignant retrobulbar lymphoma was diagnosed after repetitive intraocular lens dislocation to the anterior chamber.
CASE SUMMARY
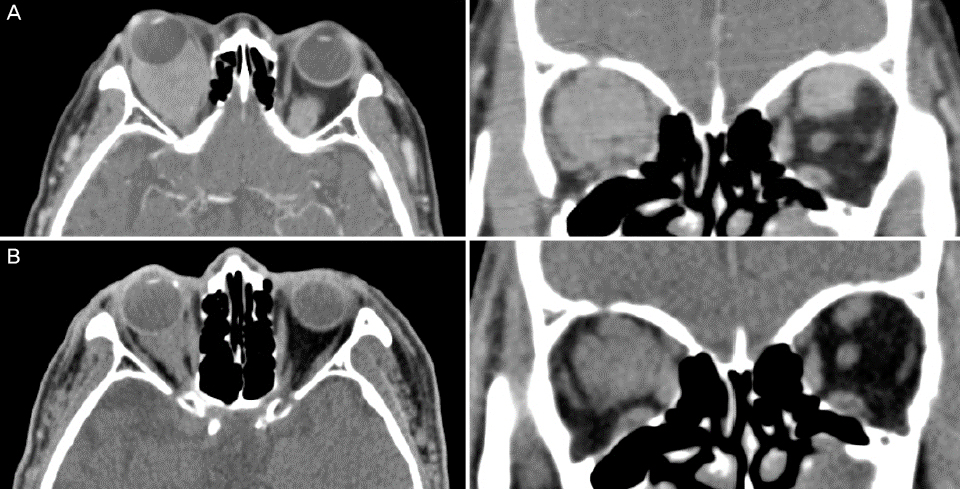
An 85-year-old male with a history of stroke who had undergone cataract surgery 10 years ago at another hospital presented with repeated intraocular lens (IOL) dislocations of both eyes into the anterior chamber. He had previously undergone IOL scleral fixation once in his left eye and twice in his right eye, but IOL dislocation was still repeatedly occurring. The best-corrected visual acuity was 0.4 in both eyes. Hertel exophthalmetry was 20 mm in his right eye and 18 mm in his left eye. Painless limitation of motion at supraduction was observed in the right eye. Funduscopy showed newly appeared choroidal folding in the right eye, so orbital computed tomography (CT) with contrast was performed. The CT scans showed bilateral homogenously enhancing retrobulbar masses. Biopsy of the masses showed a MALToma. After radiation therapy, the choroidal folds resolved and exophthalmetry improved to 10 mm in both eyes. No additional IOL dislocation occurred. During 2.5 years of follow-up, there was no evidence of recurrence or distant metastasis of the MALToma.
CONCLUSIONS
Orbital lymphomas can cause lid edema, exophthalmos, strabismus, and diplopia, and can be diagnosed with imaging modalities such as CT. Final diagnosis involves biopsy and radiation therapy or chemotherapy. If IOL dislocation occurs repeatedly, it may result from an increase in retrobulbar pressure, and concurrent choroidal folding using funduscopy is strongly recommended for imaging to check for the presence of retrobulbar masses.
MeSH Terms
Figure
Reference
-
1. Suh CO, Shim SJ, Lee SW, et al. Orbital marginal zone B-cell lymphoma of MALT: radiotherapy results and clinical behavior. Int J Radiat Oncol Biol Phys. 2006; 65:228–233.2. Lee JL, Kim MK, Lee KH, et al. Extranodal marginal zone B-cell lymphomas of mucosa-associated lymphoid tissue-type of the orbit and ocular adnexa. Ann Hematol. 2005; 84:13–18.3. Uno T, Isobe K, Shikama N, et al. Radiotherapy for extranodal, marginal zone, B-cell lymphoma of mucosa-associated lymphoid tissue originating in the ocular adnexa: a multiinstitutional, retrospective review of 50 patients. Cancer. 2003; 98:865–871.4. Le QT, Eulau SM, George TI, et al. Primary radiotherapy for localized orbital MALT lymphoma. Int J Radiat Oncol Biol Phys. 2002; 52:657–663.5. Ejima Y, Sasaki R, Okamoto Y, et al. Ocular adnexal mucosa-associated lymphoid tissue lymphoma treated with radiotherapy. Radiother Oncol. 2006; 78:6–9.6. Tsang RW, Gospodarowicz MK, Pintilie M, et al. Localized mucosa-associated lymphoid tissue lymphoma treated with radiation therapy has excellent clinical outcome. J Clin Oncol. 2003; 21:4157–4164.7. Shields JA, Bakewell B, Augsburger JJ, Flanagan JC. Classification and incidence of space-occupying lesions of the orbit. A survey of 645 biopsies. Arch Ophthalmol. 1984; 102:1606–1611.8. Carbone PP, Kaplan HS, Musshoff K, et al. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 1971; 31:1860–1861.9. Liesegang TJ. Ocular adnexal lymphoproliferative lesions. Mayo Clin Proc. 1993; 68:1003–1010.10. Coupland SE, Krause L, Delecluse HJ, et al. Lymphoproliferative lesions of the ocular adnexa. Analysis of 112 cases. Ophthalmology. 1998; 105:1430–1441.11. Hardman-Lea S, Kerr-Muir M, Wotherspoon AC, et al. Mucosalassociated lymphoid tissue lymphoma of the conjunctiva. Arch Ophthalmol. 1994; 112:1207–1212.12. Strauss EC, Warren JF, Margolis TP, Holsclaw DS. Diagnosis of conjunctival B-cell lymphoma by polymerase chain reaction heteroduplex analysis. Am J Ophthalmol. 2003; 136:207–209.13. Hayashi K, Hirata A, Hayashi H. Possible predisposing factors for in-the-bag and out-of-the-bag intraocular lens dislocation and outcomes of intraocular lens exchange surgery. Ophthalmology. 2007; 114:969–975.14. Kristianslund O, Råen M, Østern AE, Drolsum L. Late in-the-bag intraocular lens dislocation: a randomized clinical trial comparing lens repositioning and lens exchange. Ophthalmology. 2017; 124:151–159.15. Gass JD. Radial chorioretinal folds. A sign of choroidal neovascularization. Arch Ophthalmol. 1981; 99:1016–1018.16. Kowal L, Georgievski Z. Choroidal folds in Graves' ophthalmopathy. Aust N Z J Ophthalmol. 1994; 22:216.17. Singh G, Guthoff R, Foster CS. Observations on long-term follow-up of posterior scleritis. Am J Ophthalmol. 1986; 101:570–575.18. Haruyama M, Yuzawa M, Kawamura A, et al. Indocyanine green angiographic findings of chorioretinal folds. Jpn J Ophthalmol. 2001; 45:293–300.19. Hedges TR Jr, Leopold IH. Parallel retinal folds; their significance in orbital space-taking lesions. Arch Ophthalmol. 1959; 62:353–355.20. Khairallah M, Ladjimi A, Messaoud R, et al. Sectorial choroidal ischemia associated with ipsilateral lacrimal gland tumor. Am J Ophthalmol. 1997; 124:263–265.21. Fannin LA, Schiffman JC, Budenz DL. Risk factors for hypotony maculopathy. Ophthalmology. 2003; 110:1185–1191.22. Friberg TR. The etiology of choroidal folds. A biomechanical explanation. Graefes Arch Clin Exp Ophthalmol. 1989; 227:459–464.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mucosa-Associated Lymphoid Tissue Lymphoma of the Esophagus Coexistent with Bronchus-Associated Lymphoid Tissue Lymphoma of the Lung
- A case report of the Pulmonary Malignant Lymphomaof the mucosa-associated lymphoid tissue(MALT)
- A Case of Primary Pulmonary Extranodal Marginal Zone B-Cell Lymphoma of the MALT Type
- A Case of Mucosa-Associated Lymphoid Tissue Lymphoma of Colon as Multiple Large Polypoid Lesions
- A Case of Gastroduodenal Mucosa-associated Lymphoid Tissue Lymphoma Regression after Eradication of Helicobacter pylori