Asia Pac Allergy.
2015 Oct;5(4):222-229. 10.5415/apallergy.2015.5.4.222.
Pilot study of the use of Yin Qiao San in children with conventional antipyretic hypersensitivity
- Affiliations
-
- 1Department of Paediatric Medicine, Allergy Service, KK Women's and Children's Hospital, Singapore 229899, Singapore. loh.wenyin@singhealth.com.sg
- 2Allergy and Clinical Immunology Unit, Sheba Medical Center, Tel Hashomer 5262100, Israel.
- KMID: 2397039
- DOI: http://doi.org/10.5415/apallergy.2015.5.4.222
Abstract
- BACKGROUND
Children with a diagnosis of cross-reactive hypersensitivity to both paracetamol and nonsteroidal anti-inflammatory drugs are limited in their choice of antipyretics.
OBJECTIVE
The aim of this pilot study is to evaluate the feasibility of using a Chinese proprietary medicine, Yin Qiao San (YQS), for fever relief.
METHODS
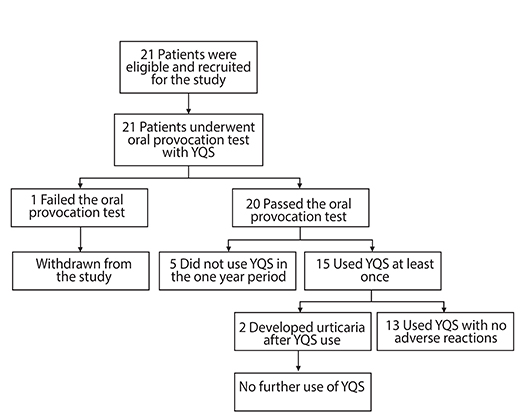
A single centre, open label, prospective clinical trial exploring the tolerability and feasibility of using YQS for fever relief in children who are unable to use conventional antipyretic medications. Children between 1-18 years of age with hypersensitivity to multiple antipyretics were recruited. Eligible participants underwent an oral provocation test with YQS. Children who passed the oral provocation test were instructed to take a prescribed dose of YQS when the temperature was >38.0℃ and continued till the fever settled. Time taken for fever resolution and any adverse events were collected.
RESULTS
A total of 21 children, mean age 10.7 years, had a diagnosis of paracetamol and ibuprofen hypersensitivity. All except one patient successfully tolerated an oral challenge of YQS. Of the 88 doses of YQS taken for fever over 38.0℃, 16 (18%) had documented temperature reduction 2 hours after ingestion and 30 (34%) had documented temperature reduction 4 hours after ingestion. There were 2 reports of urticaria after YQS use which were attributed to flare of recurrent spontaneous urticaria during the illness. None of the patients developed symptoms of circulatory compromise or respiratory distress.
CONCLUSION
YQS is generally well tolerated in patients with paracetamol and ibuprofen hypersensitivity.
Keyword
MeSH Terms
-
Acetaminophen
Anti-Inflammatory Agents, Non-Steroidal
Antipyretics
Asian Continental Ancestry Group
Child*
Cyclooxygenase 1
Diagnosis
Eating
Fever
Herbal Medicine
Humans
Hypersensitivity*
Ibuprofen
Pilot Projects*
Prospective Studies
Urticaria
Acetaminophen
Anti-Inflammatory Agents, Non-Steroidal
Antipyretics
Cyclooxygenase 1
Ibuprofen
Figure
Cited by 1 articles
-
Great learning, much networking, and friendship
Yoon-Seok Chang
Asia Pac Allergy. 2015;5(4):191-192. doi: 10.5415/apallergy.2015.5.4.191.
Reference
-
1. Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002; 99:13926–13931.
Article2. Roberts LJ, Morrow JD. Analgesic-antipyretic and antiinflammatory agents and drugs employed in the treatment of gout. In : Goodman LS, Hardman JG, Limbird LE, Gilman AG, editors. The pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill;2001. p. 687–731.3. Szczeklik A, Nizankowska E, Sanak M. Hypersensitivity to aspirin and non-steroidal antiinflammtory drugs. In : Adkinson NF, Busse WW, Bochner BS, Holgate ST, Simons FE, Lemanske RF, editors. Middelton's allergy, principles and practice. 7th ed. Philadelphia: Mosby;2009. p. 1227–1243.4. Tan VA, Gerez IF, Van Bever HP. Prevalence of drug allergy in Singaporean children. Singapore Med J. 2009; 50:1158–1161.5. Jenkins C, Costello J, Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. BMJ. 2004; 328:434.
Article6. Boussetta K, Ponvert C, Karila C, Bourgeois ML, Blic J, Scheinmann P. Hypersensitivity reactions to paracetamol in children: a study of 25 cases. Allergy. 2005; 60:1174–1177.
Article7. Stevenson DD, Szczeklik A. Clinical and pathologic perspectives on aspirin sensitivity and asthma. J Allergy Clin Immunol. 2006; 118:773–786.
Article8. Kim YP, Lee EB, Kim SY, Li D, Ban HS, Lim SS, Shin KH, Ohuchi K. Inhibition of prostaglandin E2 production by platycodin D isolated from the root of Platycodon grandiflorum. Planta Med. 2001; 67:362–364.
Article9. Wang C, Schuller Levis GB, Lee EB, Levis WR, Lee DW, Kim BS, Park SY, Park E. Platycodin D and D3 isolated from the root of Platycodon grandiflorum modulate the production of nitric oxide and secretion of TNF-alpha in activated RAW 264.7 cells. Int Immunopharmacol. 2004; 4:1039–1049.10. Wu HZ, Luo J, Yin YX, Wei Q. Effects of chlorogenic acid, an active compound activating calcineurin, purified from Flos Lonicerae on macrophage. Acta Pharmacol Sin. 2004; 25:1685–1689.11. Cho MK, Jang YP, Kim YC, Kim SG. Arctigenin, a phenylpropanoid dibenzylbutyrolactone lignan, inhibits MAP kinases and AP-1 activation via potent MKK inhibition: the role in TNF-alpha inhibition. Int Immunopharmacol. 2004; 4:1419–1429.12. Lin CC, Lu JM, Yang JJ, Chuang SC, Ujiie T. Anti-inflammatory and radical scavenge effects of Arctium lappa. Am J Chin Med. 1996; 24:127–137.
Article13. Yu LZ, Wu JY, Luo JB, Huang XG, Shao HX, Lin H. Experimental study on anti-pyretic effect of gegen qin lian decoction and its compounds. Zhongguo Zhong Yao Za Zhi. 2004; 29:663–666.14. Jin UH, Lee JY, Kang SK, Kim JK, Park WH, Kim JG, Moon SK, Kim CH. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005; 77:2760–2769.
Article15. Yuan X, Koh HL, Chui WK. A high performance liquid chromatography method for the simultaneous determination of arctiin, chlorogenic acid and glycyrrhizin in a Chinese proprietary medicine. J Pharm Biomed Anal. 2005; 39:697–704.
Article16. Yu BS, Yan XP, Xiong J, Xin Q. Simultaneous determination of chlorogenic acid, forsythin and arctiin in Chinese traditional medicines preparation by reversed phase-HPLC. Chem Pharm Bull (Tokyo). 2003; 51:421–424.
Article17. Kong XT, Fang HT, Jiang GQ, Zhai SZ, O'Connell DL, Brewster DR. Treatment of acute bronchiolitis with Chinese herbs. Arch Dis Child. 1993; 68:468–471.
Article18. Wu T, Chen X, Duan X, Juan N, Liu G, Qiao J, Wang Q, Wei J, Zhen J, Zhou L. Chinese medicinal herbs for acute bronchitis. Cochrane Database Syst Rev. 2005; (3):CD004560.
Article19. Chen XY, Wu TX, Liu GJ, Wang Q, Zheng J, Wei J, Ni J, Zhou LK, Duan X, Qiao JQ. Chinese medicinal herbs for influenza. Cochrane Database Syst Rev. 2005; (1):CD004559.
Article20. Kidon MI, Kang LW, Chin CW, Hoon LS, See Y, Goh A, Lin JT, Chay OM. Early presentation with angioedema and urticaria in cross-reactive hypersensitivity to nonsteroidal antiinflammatory drugs among young, Asian, atopic children. Pediatrics. 2005; 116:e675–e680.
Article21. Chay OM, Tang JPL, Gu K, Goh A, Phua KB, Lim WH. Use of herbal therapy among parents: a questionnaire survey. Singapore Paediatr J. 2001; 43:37–42.22. Kidon MI, Liew WK, Chiang WC, Lim SH, Goh A, Tang JP, Chay OM. Hypersensitivity to paracetamol in Asian children with early onset of nonsteroidal anti-inflammatory drug allergy. Int Arch Allergy Immunol. 2007; 144:51–56.
Article23. Woessner KM, Simon RA, Stevenson DD. The safety of celecoxib in patients with aspirin-sensitive asthma. Arthritis Rheum. 2002; 46:2201–2206.
Article24. Martin-Garcia C, Hinojosa M, Berges P, Camacho E, Garci a-Rodriguez R, Alfaya T, Iscar A. Safety of a cyclooxygenase-2 inhibitor in patients with aspirin-sensitive asthma. Chest. 2002; 121:1812–1817.
Article25. Sanchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for nonsteroidal anti-inflammatory drug sensitivity. Ann Allergy Asthma Immunol. 2000; 84:101–106.
Article26. Rachelefsky GS, Coulson A, Siegel SC, Stiehm ER. Aspirin intolerance in chronic childhood asthma: Detected by oral challenge. Pediatrics. 1975; 56:443–448.
Article27. Wald ER, Guerra N, Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991; 87:129–133.
Article28. Thompson M, Vodicka TA, Blair PS, Buckley DI, Heneghan C, Hay AD. TARGET Programme Team. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ. 2013; 347:f7027.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of San-Yin-Jiao(SP6) Acupressure on Labor Pain, Delivery Time in Women during Labor
- Effects of treatment with San-Yin-Jian(SP-6) acupressure for labor women on labor pain, length time for delivery and anxiety: A clinical trial pilot study
- Two cases of hypersensitivity to isopropylantipyrine
- Antinociceptive, Immunomodulatory and Antipyretic Activity of Nymphayol Isolated from Nymphaea stellata (Willd.) Flowers
- A Comparative Study on the Applied Effects of Auricular Acupressure Therapy on Insomnia in the Elderly by Sasangin Constitution: Based on Tae Yin In, So Yang In, and So Yin In