Cancer Res Treat.
2017 Oct;49(4):1033-1043. 10.4143/crt.2016.413.
Korean Cancer Patients' Awareness of Clinical Trials, Perceptions on the Benefit and Willingness to Participate
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. moisa@snu.ac.kr
- 2Translational Medicine Major, Department of Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea.
- KMID: 2394822
- DOI: http://doi.org/10.4143/crt.2016.413
Abstract
- PURPOSE
The purpose of this study was to assess current levels of awareness of clinical trials (CTs), perceptions regarding their benefits and willingness to participate to CTs among Korean cancer patients.
MATERIALS AND METHODS
From December 2012 to August 2015, we distributed questionnaires to cancer patients receiving systemic anti-cancer therapy at Seoul National University Hospital, Seoul, Korea.
RESULTS
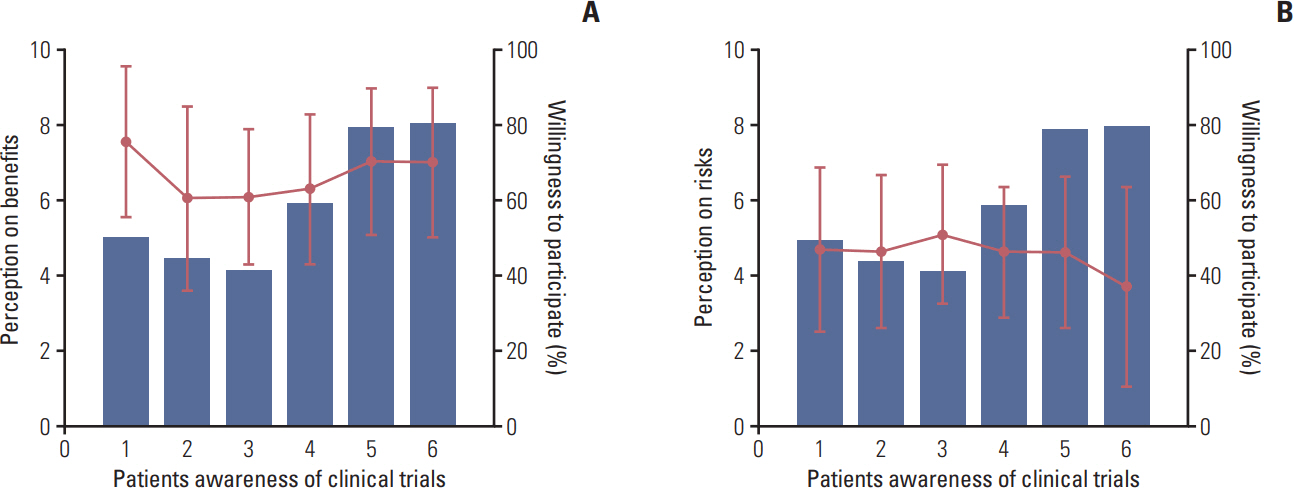
A total of 397 out of 520 requested patients (76.3%) responded to the survey. Among the 397 patients, 62.5% were female and the median age was 52 years. Overall, 97.4% (387/397) answered that they have at least heard of CTs. When asked about their level of awareness, 23.8% (92/387) answered that they could more than roughly explain about CTs. The average visual analogue scale score of CT benefit in all patients was 6.43 (standard deviation, 2.20). Patients who were only familiar with the term without detailed knowledge of the contents had the least expectation of benefit from CTs (p=0.015). When asked about their willingness to participate in CTs, 56.7% (225/397) answered positively. Patients with higher levels of awareness of CTs showed higher willingness to participate (p < 0.001). Heavily treated patients and patients with previous experience regarding CTs also showed a higher willingness to participate (p < 0.001). The perceived benefit of CTs was higher in the group willing to participate (p=0.026).
CONCLUSION
The patient's level of awareness regarding CTs was positively related to the positive perception and willingness to participate. Although the general awareness of CTs was high, a relatively large proportion of patients did not have accurate knowledge; therefore, proper and accurate patient education is necessary.
Keyword
Figure
Cited by 1 articles
-
Perception and Satisfaction of Anticancer Drug Clinical Trials in Cancer Patients
Ju Kyung Jeon, Jeong Hye Kim
Asian Oncol Nurs. 2019;19(1):18-26. doi: 10.5388/aon.2019.19.1.18.
Reference
-
References
1. CenterWatch. FDA approved drugs for oncology [Internet]. Boston, MA: CenterWatch;c2016. [cited 2016 Jun 28]. Available from: https://www.centerwatch.com/drug-information/fdaapproved-drugs/therapeutic-area/12/oncology.2. ClinicalTrals.gov. Trends, charts, and maps [Internet]. Bethesda, MD: U.S. National Institutes of Health;c2016. [cited 2016 Jun 28]. Available from: https://clinicaltrials.gov/ct2/resources/trends.3. Caldwell PH, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS Med. 2010; 7:e1000368.
Article4. Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999; 52:1143–56.5. Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008; 112:228–42.
Article6. Kim JW, Kim SJ, Chung YH, Kwon JH, Lee HJ, Chung YJ, et al. Cancer patients' awareness of clinical trials, perceptions on the benefit and willingness to participate: Korean perspectives. Br J Cancer. 2008; 99:1593–9.
Article7. Trading Economics. South Korea GDP [Internet]. New York, NY: Trading Economics;c2016. [cited 2016 Jun 28]. Available from: http://www.tradingeconomics.com/south-korea/gdp.8. Shim BY, Park SH, Lee S, Kim JS, Lee KE, Kang YK, et al. Current status and challenges of cancer clinical trials in Korea. Cancer Res Treat. 2016; 48:20–7.
Article9. Tejeda HA, Green SB, Trimble EL, Ford L, High JL, Ungerleider RS, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996; 88:812–6.
Article10. Lovato LC, Hill K, Hertert S, Hunninghake DB, Probstfield JL. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997; 18:328–52.
Article11. Haidich AB, Ioannidis JP. Effect of early patient enrollment on the time to completion and publication of randomized controlled trials. Am J Epidemiol. 2001; 154:873–80.
Article12. Ehrlich PF, Newman KD, Haase GM, Lobe TE, Wiener ES, Holcomb GW. Lessons learned from a failed multi-institutional randomized controlled study. J Pediatr Surg. 2002; 37:431–6.
Article13. Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003; 21:830–5.
Article14. Sood A, Prasad K, Chhatwani L, Shinozaki E, Cha SS, Loehrer LL, et al. Patients' attitudes and preferences about participation and recruitment strategies in clinical trials. Mayo Clin Proc. 2009; 84:243–7.
Article15. Wallington SF, Luta G, Noone AM, Caicedo L, Lopez-Class M, Sheppard V, et al. Assessing the awareness of and willingness to participate in cancer clinical trials among immigrant Latinos. J Community Health. 2012; 37:335–43.
Article16. Li JY, Yu CH, Jiang Y. Participation in cancer clinical trials as viewed by Chinese patients and their families. Oncology. 2010; 79:343–8.
Article17. Bazarbashi S, Hassan A, Eldin AM, Soudy H, Hussain F. Awareness and perceptions of clinical trials in cancer patients and their families in Saudi Arabia. J Cancer Educ. 2015; 30:655–9.
Article18. Lara PN Jr, Paterniti DA, Chiechi C, Turrell C, Morain C, Horan N, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005; 23:9282–9.
Article19. Lee SJ, Park LC, Lee J, Kim S, Choi MK, Hong JY, et al. Unique perception of clinical trials by Korean cancer patients. BMC Cancer. 2012; 12:594.
Article20. Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013; 9:267–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Restaurateur's Willingness to Participate in the Healthy Restaurant Program in Seoul
- Knowledge and Perception about Clinical Research Shapes Behavior: Face to Face Survey in Korean General Public
- Analysis of Subgroups with Lower Level of Patient Safety Perceptions Using Decision-Tree Analysis
- Awareness of and Attitudes toward Human Papillomavirus Vaccination among Adults in Korea: 9-Year Changes in Nationwide Surveys
- Patient Attitudes and Considerations in Decision toward Participation in Clinical Trials of Chronic Diseases