Korean J Obstet Gynecol.
2012 Oct;55(10):777-781.
A case of primitive neuroectodermal tumor of the ovary
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. garden.lee@samsung.com
Abstract
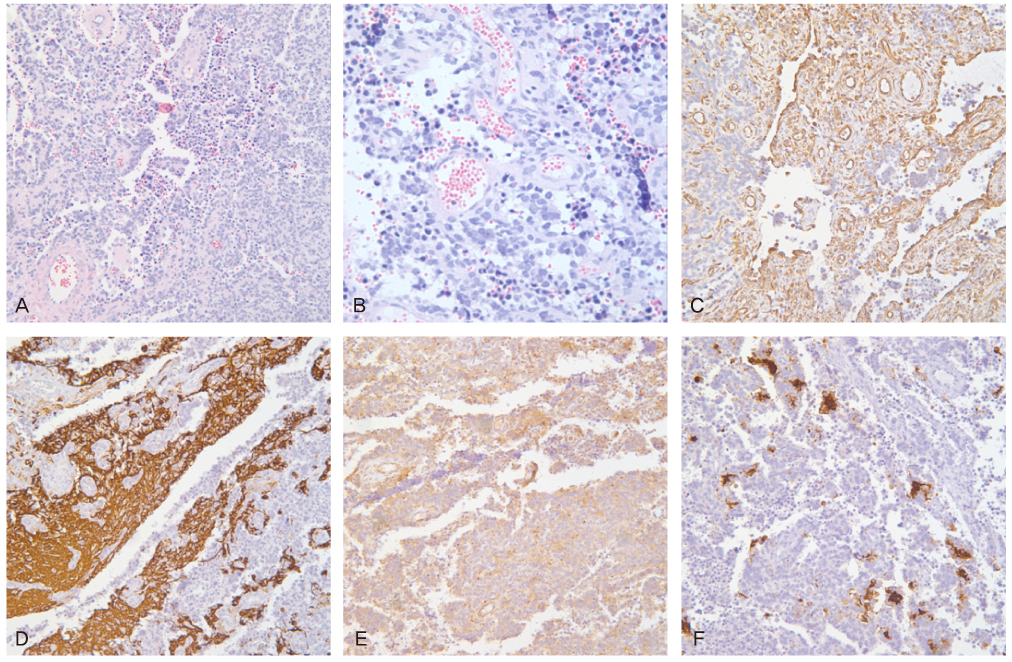
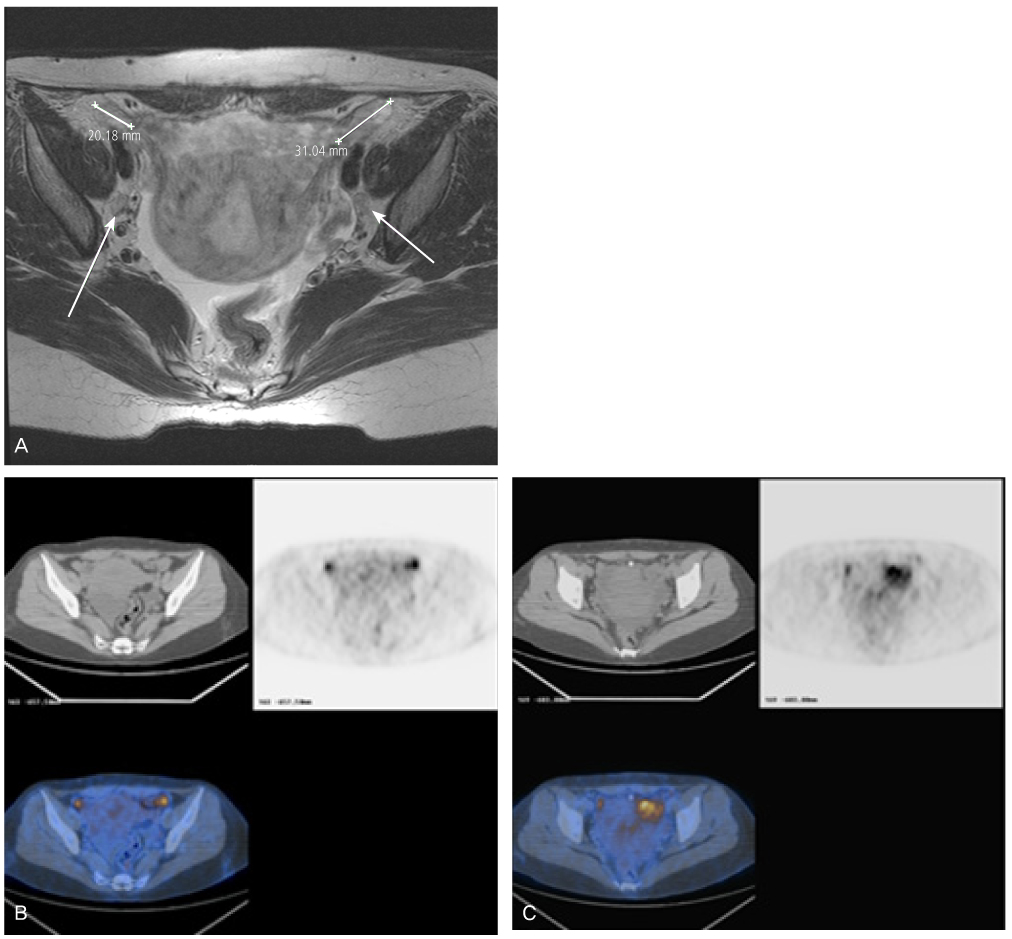
- Peripheral primitive neuroectodermal tumor (PNET) is a small round tumor belonging to the PNET/Ewing's sarcoma family classified based on location in the body. There have been a small number of case reports of PNET arising in the ovary. We present extremely rare case of PNET of the ovary occurring in a 32-year-old pregnant woman, which was detected during her second Cesarean section. She had a past history of oophorectomy due to a huge mature solid teratoma in left ovary during her first Cesarean section. She got the operation of right salpingoophorectomy during the second Cesarean section at local gynecologic clinic and referred to our institute. Magnetic resonance imaging and positron emission tomography revealed the PNET was metastased to the peritoneum and the lymph nodes. We had persecuted vincristine, doxorubicin, cyclophosphamide, and etoposide/ifosfamide 8 cycles. There has been no evidence of local tumor recurrence and metastasis after the chemotherapy until now.
MeSH Terms
-
Adult
Cesarean Section
Cyclophosphamide
Doxorubicin
Female
Humans
Lymph Nodes
Magnetic Resonance Imaging
Neoplasm Metastasis
Neuroectodermal Tumors, Primitive
Ovariectomy
Ovary
Peritoneum
Positron-Emission Tomography
Pregnancy
Pregnant Women
Recurrence
Sarcoma
Teratoma
Vincristine
Cyclophosphamide
Doxorubicin
Vincristine
Figure
Reference
-
1. Dehner LP. Primitive neuroectodermal tumor and Ewing's sarcoma. Am J Surg Pathol. 1993. 17:1–13.2. Kim KJ, Jang BW, Lee SK, Kim BK, Nam SL. A case of peripheral primitive neuroectodermal tumor of the ovary. Int J Gynecol Cancer. 2004. 14:370–372.3. Katz RL, Quezado M, Senderowicz AM, Villalba L, Laskin WB, Tsokos M. An intra-abdominal small round cell neoplasm with features of primitive neuroectodermal and desmoplastic round cell tumor and a EWS/FLI-1 fusion transcript. Hum Pathol. 1997. 28:502–509.4. Marley EF, Liapis H, Humphrey PA, Nadler RB, Siegel CL, Zhu X, et al. Primitive neuroectodermal tumor of the kidney: another enigma: a pathologic, immunohistochemical, and molecular diagnostic study. Am J Surg Pathol. 1997. 21:354–359.5. Demirtas E, Guven S, Guven ES, Baykal C, Ayhan A. Two successful spontaneous pregnancies in a patient with a primary primitive neuroectodermal tumor of the ovary. Fertil Steril. 2004. 81:679–681.6. Hoover K, Jenkins TR. Evaluation and management of adnexal mass in pregnancy. Am J Obstet Gynecol. 2011. 205:97–102.7. Leiserowitz GS. Managing ovarian masses during pregnancy. Obstet Gynecol Surv. 2006. 61:463–470.8. Kumari I, Kaur S, Mohan H, Huria A. Adnexal masses in pregnancy: a 5-year review. Aust N Z J Obstet Gynaecol. 2006. 46:52–54.9. Ateser G, Yildiz O, Leblebici C, Mandel NM, Unal F, Turna H, et al. Metastatic primitive neuroectodermal tumor of the ovary in pregnancy. Int J Gynecol Cancer. 2007. 17:266–269.10. Morovic A, Damjanov I. Neuroectodermal ovarian tumors: a brief overview. Histol Histopathol. 2008. 23:765–771.11. Takano T, Akahira J, Moriya T, Murakami T, Tanaka M, Goto M, et al. Primary ependymoma of the ovary: a case report and literature review. Int J Gynecol Cancer. 2005. 15:1138–1141.12. Hirahara F, Yamanaka M, Miyagia E, Nakazawa T, Gorai I, Minaguchi H, et al. Pure ovarian ependymoma: report of a case treated with surgery, chemotherapy, irradiation and hyperthermotherapy. Eur J Obstet Gynecol Reprod Biol. 1997. 75:221–223.13. Urano F, Umezawa A, Yabe H, Hong W, Yoshida K, Fujinaga K, et al. Molecular analysis of Ewing's sarcoma: another fusion gene, EWS-E1AF, available for diagnosis. Jpn J Cancer Res. 1998. 89:703–711.14. Kawauchi S, Fukuda T, Miyamoto S, Yoshioka J, Shirahama S, Saito T, et al. Peripheral primitive neuroectodermal tumor of the ovary confirmed by CD99 immunostaining, karyotypic analysis, and RT-PCR for EWS/FLI-1 chimeric mRNA. Am J Surg Pathol. 1998. 22:1417–1422.15. Chow SN, Lin MC, Shen J, Wang S, Jong YJ, Chien CH. Analysis of chromosome abnormalities by comparative genomic hybridization in malignant peripheral primitive neuroectodermal tumor of the ovary. Gynecol Oncol. 2004. 92:752–760.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primitive Neuroectodermal Tumor of the Ovary: A case report
- A Case of a Primitive Neuroectodermal Tumor Detected from a Duodenal Metastasis
- Primitive Neuroectodermal Tumor of Mediastinum in an Adult: A Case Report
- A case of extraosseous ewing's sarcoma/primitive neuroectodermal tumor of the ovary
- A Case of Primitive Neuroectodermal Tumor in the Nasal Cavity