J Korean Soc Radiol.
2017 Oct;77(4):222-228. 10.3348/jksr.2017.77.4.222.
Ultrasonographic Features of Complications Associated with Injectable Fillers in the Cervicofacial Region
- Affiliations
-
- 1Department of Radiology, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea. hshong@schmc.ac.kr
- 2Department of Pathology, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea.
- KMID: 2392118
- DOI: http://doi.org/10.3348/jksr.2017.77.4.222
Abstract
- PURPOSE
The aim of this study was to evaluate the ultrasonographic (US) features of complications associated with injectable fillers in the cervicofacial region.
MATERIALS AND METHODS
This retrospective study was approved by our Institutional Review Board. Thirty-nine patients (37 females and 2 males; mean age: 52 years; age range: 26-83 years), both symptomatic (n = 32) and asymptomatic (n = 7), who underwent cosmetic procedures in the cervicofacial area and US, were included.
RESULTS
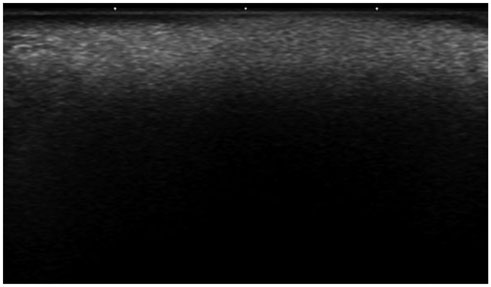
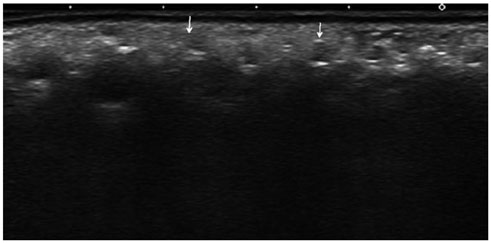
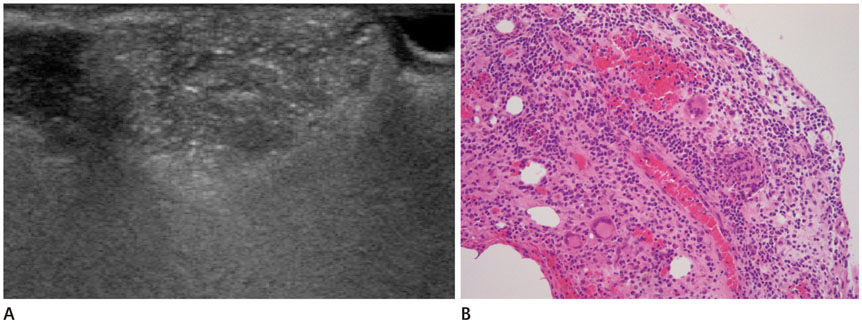
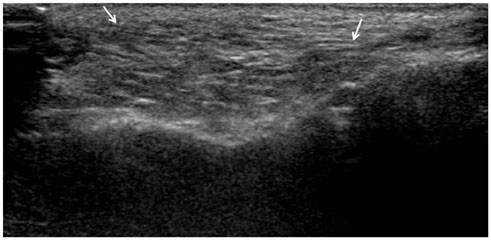
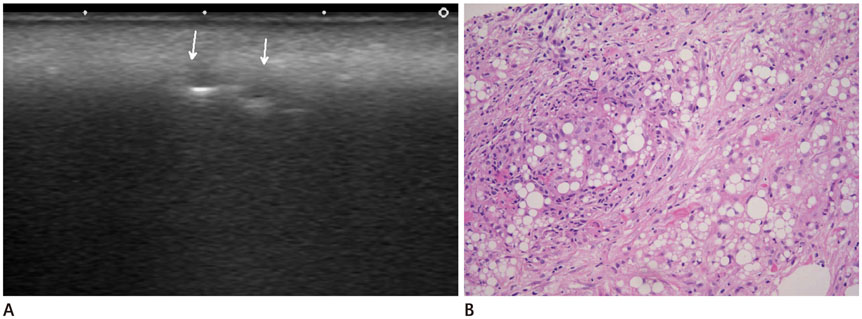
All of the cases were classified into five types based on major US features, including (the most common feature) diffuse high echogenicity with posterior snow storming (n = 17, 39%). The other types included a hypoechoic nodule with high echoic lines (n = 10, 23%), a round hypoechoic nodule (n = 12, 27%), a heterogeneously echogenic lesion with internal echoic dots (n = 1, 2%) and an ill-defined non-mass-like fatty lesion (n = 4, 9%). The time between cosmetic injection and evaluation was variable (range: 3 weeks-21 years). Usually, the injected material was either unknown or illegal (n = 24). Ten patients underwent pathological evaluation. Many histological types of foreign body granulomas were evident.
CONCLUSION
Knowledge of the US features of complications associated with fillers may reduce the likelihood of misinterpreting the injected area as a true lesion, in turn decreasing unnecessary invasive procedures and costs for patients.
MeSH Terms
Figure
Reference
-
1. Kontis TC, Rivkin A. The history of injectable facial fillers. Facial Plast Surg. 2009; 25:67–72.2. Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther. 2009; 11:240–247.3. Ginat DT, Schatz CJ. Imaging features of midface injectable fillers and associated complications. AJNR Am J Neuroradiol. 2013; 34:1488–1495.4. Christensen L, Breiting V, Janssen M, Vuust J, Hogdall E. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg. 2005; 29:34–48.5. Lombardi T, Samson J, Plantier F, Husson C, Küffer R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J Oral Pathol Med. 2004; 33:115–120.6. Dayan SH, Bassichis BA. Facial dermal fillers: selection of appropriate products and techniques. Aesthet Surg J. 2008; 28:335–347.7. Jham BC, Nikitakis NG, Scheper MA, Papadimitriou JC, Levy BA, Rivera H. Granulomatous foreign-body reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009; 67:280–285.8. Wortsman X, Wortsman J, Orlandi C, Cardenas G, Sazunic I, Jemec GB. Ultrasound detection and identification of cosmetic fillers in the skin. J Eur Acad Dermatol Venereol. 2012; 26:292–301.9. Young SR, Bolton PA, Downie J. Use of high-frequency ultrasound in the assessment of injectable dermal fillers. Skin Res Technol. 2008; 14:320–323.10. Mastruserio DN, Pesqueira MJ, Cobb MW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996; 34(5 Pt 1):849–852.11. Maly A, Regev E, Meir K, Maly B. Tissue reaction to liquid silicone simulating low-grade liposarcoma following lip augmentation. J Oral Pathol Med. 2004; 33:314.12. Morhenn VB, Lemperle G, Gallo RL. Phagocytosis of different particulate dermal filler substances by human macrophages and skin cells. Dermatol Surg. 2002; 28:484–490.13. Shafir R, Amir A, Gur E. Long-term complications of facial injections with Restylane (injectable hyaluronic acid). Plast Reconstr Surg. 2000; 106:1215–1216.14. Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001; 23:197–202.15. Verpaele A, Strand A. Restylane SubQ, a non-animal stabilized hyaluronic acid gel for soft tissue augmentation of the mid- and lower face. Aesthet Surg J. 2006; 26:S10–S17.16. Reda-Lari A. Augmentation of the malar area with polyacrylamide hydrogel: experience with more than 1300 patients. Aesthet Surg J. 2008; 28:131–138.17. Sturm LP, Cooter RD, Mutimer KL, Graham JC, Maddern GJ. A systematic review of dermal fillers for age-related lines and wrinkles. ANZ J Surg. 2011; 81:9–17.18. Judodihardjo H, Dykes P. Objective and subjective measurements of cutaneous inflammation after a novel hyaluronic acid injection. Dermatol Surg. 2008; 34:Suppl 1. S110–S114.19. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009; 123:1842–1863.20. Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006; 118:3 Suppl. 92S–107S.21. Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with aldara (imiquimod 5%). Dermatol Surg. 2003; 29:429–443.22. Rapaport MJ, Vinnik C, Zarem H. Injectable silicone: cause of facial nodules, cellulitis, ulceration, and migration. Aesthetic Plast Surg. 1996; 20:267–276.23. Hirsch RJ, Stier M. Complications of soft tissue augmentation. J Drugs Dermatol. 2008; 7:841–845.24. Schelke LW, Van Den Elzen HJ, Erkamp PP, Neumann HA. Use of ultrasound to provide overall information on facial fillers and surrounding tissue. Dermatol Surg. 2010; 36:Suppl 3. 1843–1851.25. Wortsman X, Wortsman J. Clinical usefulness of variable-frequency ultrasound in localized lesions of the skin. J Am Acad Dermatol. 2010; 62:247–256.26. Kuwano Y, Ishizaki K, Watanabe R, Nanko H. Efficacy of diagnostic ultrasonography of lipomas, epidermal cysts, and ganglions. Arch Dermatol. 2009; 145:761–764.27. Fischer J, Metzler G, Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007; 143:507–510.28. Feeney JN, Fox JJ, Akhurst T. Radiological impact of the use of calcium hydroxylapatite dermal fillers. Clin Radiol. 2009; 64:897–902.29. Requena L, Requena C, Christensen L, Zimmermann US, Kutzner H, Cerroni L. Adverse reactions to injectable soft tissue fillers. J Am Acad Dermatol. 2011; 64:1–34. quiz 35-36.30. Dadzie OE, Mahalingam M, Parada M, El Helou T, Philips T, Bhawan J. Adverse cutaneous reactions to soft tissue fillers--a review of the histological features. J Cutan Pathol. 2008; 35:536–548.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A revision and summary of injectable fillers
- Management of a Visible Nodule Following Poly-L-Lactic Acid Injection in the Periorbital Area
- Long-Term Side Effects of Soft Tissue Filler Injection
- Remote migration of breast filler to the inguinal area: a case report
- Late-Onset Complication of Fillers: Paraffinoma of the Lower Eyelids Clinically Mimicking Xanthelasma