J Korean Endocr Soc.
2006 Dec;21(6):542-547.
A Case of Adult Onset Type II Citrullinemia with SLC25A13 Gene Mutation
- Affiliations
-
- 1Division of Endocrinology, Department of Internal Medicine, College of Medicine, Chosun University, Korea.
- 2Green Cross Reference Laboratory, Korea.
Abstract
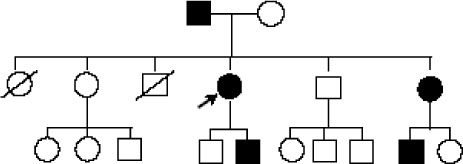
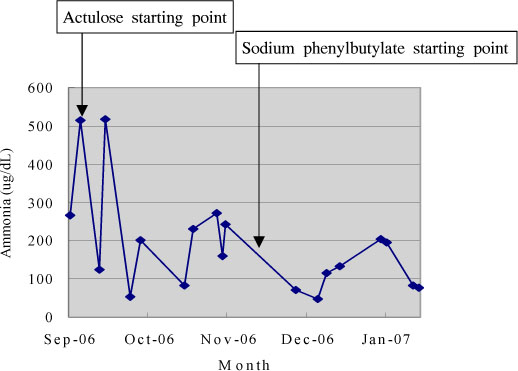
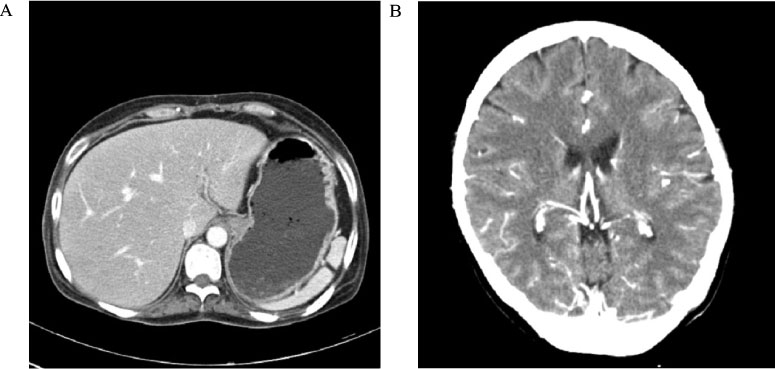
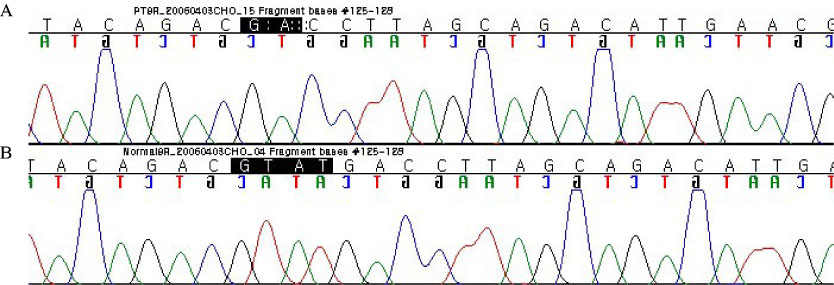
- Adult onset type II citrullinemia is an autosomal recessive disorder of the amino acid metabolism caused by a deficiency of liver specific argininosuccinate synthetase activity. This disease can occur at any age in life with recurrent episodes of neurological signs and symptoms such as disorientation, abnormal behaviors (aggression, irritability and hyperactivity), seizures, coma and potential death from brain edema, which are resulted from hyperammonemia. We should consider this rare metabolic disease for the adult patient who exhibits mental change and hyperammonemia without liver or brain disease. Recently. SLC25A13 gene, encoding the mitochondrial aspartate glutamate carrier protein named citrin, is demonstrated to be responsible for adult onset type II citrullinemia. We experienced a 39-year-old female who suffered from generalized weakness, dizziness and lethargy, and diagnosed as adult onset type II citrullinemia by highly elevated plasma citrulline and ammonia and the SLC25A13 gene mutation. We report here on this unusual case of adult onset type II citrullulinemia with a brief review of the related literature.
MeSH Terms
Figure
Reference
-
1. Jackson MJ, Beaudet AL, O'Brien WE. Mammalian urea cycle enzymes. Ann Rev Genet. 1986. 20:431–464.2. Saheki T, Kobayashi K, Inoue I. Hereditary disorders of the urea cycle in man: biochemical and molecular approach. Rev Physiol Biochem Pharmacol. 1987. 108:21–68.3. Iijima M, Jalil A, Begum L, Yasuda T, Yamaguchi N, Xian Li M, Kawada N, Endou H, Kobayashi K, Saheki T. Pathogenesis of adult onset type II citrullinemia caused by deficiency of citrin, a mitochondrial solute carrier protein: tissue and subcellular localization of citrin. Adv Enzyme Regul. 2001. 41:325–342.4. Kobayashi K, Sinasac DS, Iijima M, Boright AP, Begum L, Lee JR, Yasuda T, Ikeda S, Hirano R, Terazono H, Crackower MA, Kondo I, Tsui LC, Scherer SW, Saheki T. The gene mutated in adult-onset type II citrullinemia encodes a putative mitochondrial carrier protein. Nat Genet. 1999. 22:159–163.5. Kim BR, Won SM, Bang HK, Lee DH, Lee SJ. A case of citrullinemia. J Korean Pediatr Soc. 1987. 30:797–804.6. Park DS, Kim DU, Moon SW, Lee IJ. A case of citrullinemia. J Korean Pediatr Soc. 1997. 40:584–592.7. Park HJ, Lim HJ, Jung IS, Kim YH, Kim IH, Chung IK, Kim HS, Park SH, Lee MH, Kim SJ, Lee DH. A case of adult-type citrullinemia with hyperammonemia. Korean J Gastroenterol. 2002. 39:379–385.8. Kobayashi K, Shaheen N, Kumashiro R, Tanikawa K, O'Brien WE, Beaudet AL, Saheki T. A search for the primary abnormality in adult onset type II citrullinemia. Am J Hum Genet. 1993. 53:1024–1030.9. Saheki T, Kobayashi K, Iijima M, Horiuchi M, Begum L, Jalil MA, Li MX, Lu YB, Ushikai M, Tabata A, Moriyama M, Hsiao KJ, Yang Y. Adult onset type II citrullinemia and idiopathic neonatal hepatitis caused by citrin deficiency: involvement of the aspartate glutamate carrier for urea synthesis and maintenance of the urea cycle. Mol Genet Metab. 2004. 81:suppl 1. S20–S26.10. Shiohama N, Sugita Y, Imamura N, Sato T, Mizuno Y. Type II citrullinemia triggered by acetaminophen. No To Shinkei. 1993. 45:865–870.11. Okeda R, Tanaka M, Kawahara Y, Tokushige J, Imai T, Kameya K. Adult-type citrullinemia. Acta Neuropathol. 1989. 78:96–100.12. Tanaka T, Nagao M, Tsutsumi H. Application of mutation analysis for the previously uncertain cases of adult-onset type II citrullinemia (CTLN2) and their clinical profiles. Tohoku J Exp Med. 2002. 198:89–97.13. Palmieri L, Pardo B, Lasorsa FM, del Arco A, Kobayashi K, Iijima M, Runswick MJ, Walker JE, Saheki T, Satrustegui J, Palmieri F. Citrin and aralarl are Ca2+ stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001. 20:5060–5069.14. Saheki T, Kobayashi K, Inoue I, Matuo S, Hagihara S, Noda T. Increased urinary excretion of arginosuccinate in type II citrullinemia. Clin Chim Acta. 1987. 170:297–304.15. Kobayashi K, Horiuchi M, Saheki T. Pancreatic secretory trypsin inhibitor as a diagnostic marker for adult onset type II citrullinemia. Hepatology. 1997. 25:1160–1165.16. Imamura Y, Kobayashi K, Shibatou S, Aburada S, Tahara K, Kubozono O, Saheki T. Effectiveness of carbohydrate-restricted diet and arginine granules therapy for adult onset type II citrullinemia: a case report of siblings showing homozygous SLC25A13 mutation with and without the disease. Hepatol Res. 2003. 26:68–72.17. Honda S, Yamamoto K, Sekizuka M, Oshima Y, Nagai K, Hashimoto G, Kaneko H, Tomomasa T, Konno Y, Horiuchi R. Successful treatment of severe hyperammonemia using sodium phenylacetate powder prepared in hospital pharmacy. Biol Pharm Bull. 2002. 25:1244–1246.18. Ikeda S, Yazaki M, Takei Y, Ikegami T, Hashikura Y, Kawasaki S, Iwai M, Kobayashi K, Saheki T. Type II citrullinemia: clinical pictures and the therapeutic effect of liver transplantation. J Neurol Neurosurg Psychiatry. 2001. 71:663–670.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Novel Compound Heterozygote Mutations of the SLC25A13 Gene in an Infant with Neonatal-onset Type II Citrullinemia Detected by Newborn Mass Screening
- Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency in Korean Infants
- A Novel Argininosuccinate Synthetase Gene Mutation in a Korean Family with Type I Citrullinemia
- Auxiliary partial orthotopic liver transplantation for adult onset type II citrullinemia
- A Case of Citrullinemia Presenting with Status Epilepticus