Korean J Gastroenterol.
2017 Aug;70(2):81-88. 10.4166/kjg.2017.70.2.81.
Associations between Body Mass Index and Chronic Atrophic Gastritis and Intestinal Metaplasia
- Affiliations
-
- 1Department of Family Medicine, Kyungpook National University School of Medicine, Daegu, Korea. ychfm@knu.ac.kr
- 2Department of Family Medicine, Kyungpook National University Chilgok Hospital, Daegu, Korea.
- KMID: 2391365
- DOI: http://doi.org/10.4166/kjg.2017.70.2.81
Abstract
- BACKGROUND/AIMS
Chronic atrophic gastritis (AG) and intestinal metaplasia (IM) of the stomach are premalignant lesions. The present study aimed to examine the associations between obesity and these lesions.
METHODS
A total of 2,997 patients, who underwent gastroscopy, participated in this study, excluding those who had been diagnosed with gastric cancer. Participants were divided into four groups based on their body mass index (BMI). The risk of AG and IM with increasing BMI was analyzed in men and women, separately.
RESULTS
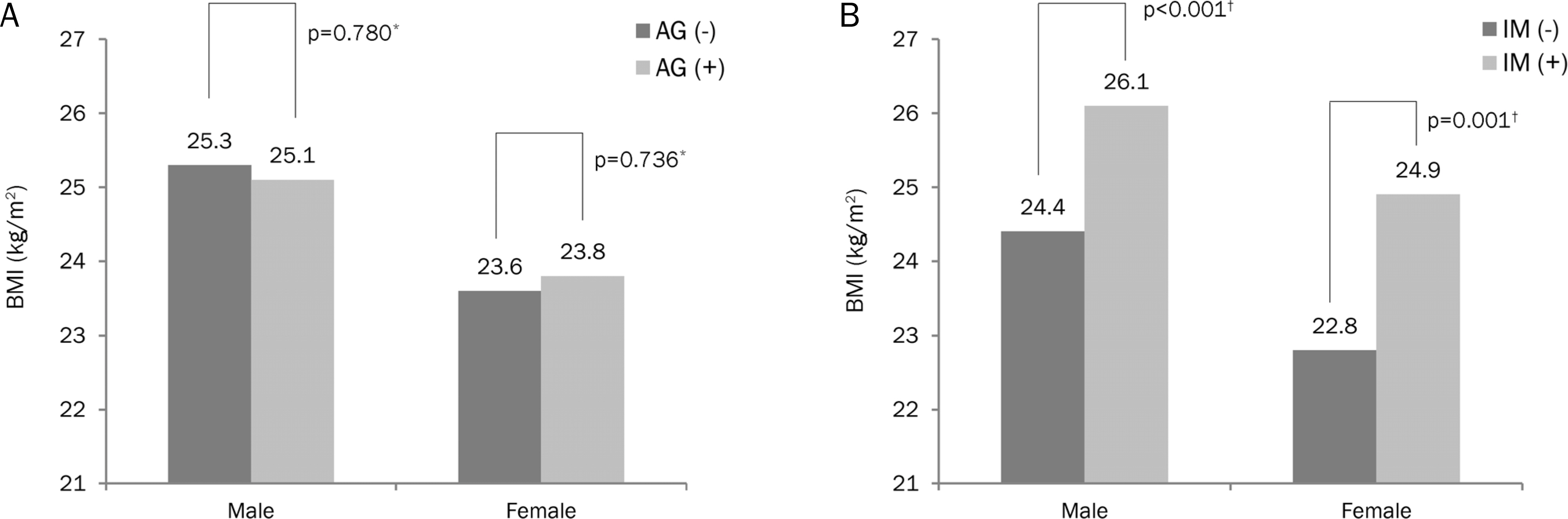
The association between BMI and AG was not significant. After adjusting for age, smoking, alcohol, and AG, the odds ratios for IM in the overweight, obesity, and severe obesity groups were 2.25 (95% confidence interval [CI], 1.50-3.37), 2.32 (95% CI, 1.58-3.42), and 4.86 (95% CI, 2.04-11.5) in men, and 2.66 (95% CI, 1.29-5.47), 4.46 (95% CI, 2.28-8.75), and 9.57 (95% CI, 3.26-28.12) in women, compared with the normal BMI group.
CONCLUSIONS
Gastric IM was significantly associated with increased BMI.
Keyword
MeSH Terms
Figure
Reference
-
References
1. The Sixth Korea National Health and Nutrition Examination Survey (KNHANES VI-2), 2014. [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2015 Dec 23. [cited 2016 Sep 21]. Available from:. https://knhanes.cdc.go.kr/knhanes/sub04/sub04_03.do?classType=7.2. Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013; 62:933–947.
Article3. Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013; 144:1252–1261.
Article4. Diet, nutrition, physical activity and stomach cancer. [Internet]. London: World Cancer Research Fund/American Institute for Cancer Research;2016. [cited 2016 Sep 21]. Available from:. http://preventcancer.aicr.org/WhjIUhch78YhhhJKl987hhHJIl-JKy67Hgg&Yh/DRAFT-CUP-STOMACH-REPORT-FINAL.pdf.5. Chen Y, Liu L, Wang X, et al. Body mass index and risk of gastric cancer: a metaanalysis of a population with more than ten million from 24 prospective studies. Cancer Epidemiol Biomarkers Prev. 2013; 22:1395–1408.
Article6. Park YH, Kim N. Review of atrophic gastritis and intestinal abdominal as a premalignant lesion of gastric cancer. J Cancer Prev. 2015; 20:25–40.7. Correa P. Human gastric carcinogenesis: a multistep and abdominal process–first American Cancer Society Award Lecture on cancer epidemiology and prevention. Cancer Res. 1992; 52:6735–6740.8. Torisu T, Matsumoto T, Takata Y, et al. Atrophic gastritis, but not antibody to Helicobacter pylori, is associated with body mass abdominal in a Japanese population. J Gastroenterol. 2008; 43:762–766.9. Watabe H, Mitsushima T, Derakhshan MH, et al. Study of association between atrophic gastritis and body mass index: a cross-sectional study in 10,197 Japanese subjects. Dig Dis Sci. 2009; 54:988–995.
Article10. Felley C, Bouzourene H, VanMelle MB, et al. Age, smoking and overweight contribute to the development of intestinal abdominal of the cardia. World J Gastroenterol. 2012; 18:2076–2083.11. Kim HJ, Kim N, Kim HY, et al. Relationship between body mass index and the risk of early gastric cancer and dysplasia regardless of Helicobacter pylori infection. Gastric Cancer. 2015; 18:762–773.
Article12. Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori abdominal and the development of gastric cancer. N Engl J Med. 2001; 345:784–789.13. Kim N, Park YS, Cho SI, et al. Prevalence and risk factors of abdominal gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter. 2008; 13:245–255.14. Joo YE, Park HK, Myung DS, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia: a nationwide multicenter prospective study in Korea. Gut Liver. 2013; 7:303–310.
Article15. Lee JY, Kim N, Lee HS, et al. Correlations among endoscopic, abdominal and serologic diagnoses for the assessment of atrophic gastritis. J Cancer Prev. 2014; 19:47–55.16. Lin BR, Shun CT, Wang TH, Lin JT. Endoscopic diagnosis of abdominal metaplasia of stomach–accuracy judged by histology. Hepatogastroenterology. 1999; 46:162–166.17. Klok MD, Jakobsdottir S, Drent ML. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev. 2007; 8:21–34.
Article18. Jun DW, Lee OY, Lee YY, Choi HS, Kim TH, Yoon BC. Correlation between gastrointestinal symptoms and gastric leptin and abdominal expression in patients with gastritis. Dig Dis Sci. 2007; 52:2866–2872.19. Roper J, Francois F, Shue PL, et al. Leptin and ghrelin in relation to Helicobacter pylori status in adult males. J Clin Endocrinol Metab. 2008; 93:2350–2357.20. Inagaki-Ohara K, Okamoto S, Takagi K, et al. Leptin receptor abdominaling is required for high-fat diet-induced atrophic gastritis in mice. Nutr Metab (Lond). 2016; 13:7.
Article21. Walker MM, Talley NJ. Review article: bacteria and pathogenesis of disease in the upper gastrointestinal tract–beyond the era of Helicobacter pylori. Aliment Pharmacol Ther. 2014; 39:767–779.22. Ohata H, Kitauchi S, Yoshimura N, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection abdominals risk of gastric cancer. Int J Cancer. 2004; 109:138–143.23. Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008; 17:352–358.
Article24. Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, abdominal, and cancer. Annu Rev Pathol. 2016; 11:421–449.25. van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and abdominal: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009; 18:2569–2578.26. Cao DZ, Sun WH, Ou XL, et al. Effects of folic acid on epithelial apoptosis and expression of Bcl-2 and p53 in premalignant abdominal lesions. World J Gastroenterol. 2005; 11:1571–1576.27. Zullo A, Rinaldi V, Hassan C, et al. Ascorbic acid and intestinal metaplasia in the stomach: a prospective, randomized study. Aliment Pharmacol Ther. 2000; 14:1303–1309.
Article28. Lee JY, Kim HY, Kim KH, et al. No changing trends in incidence of gastric cardia cancer in Korea. J Korean Med Sci. 2003; 18:53–57.
Article29. Abrams JA, Gonsalves L, Neugut AI. Diverging trends in the abdominal of reflux-related and Helicobacter pylori-related gastric cardia cancer. J Clin Gastroenterol. 2013; 47:322–327.30. Miwata T, Quach DT, Hiyama T, et al. Interobserver and intraobserver agreement for gastric mucosa atrophy. BMC Gastroenterol. 2015; 15:95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adherence of Helicobacter pylori to Areas of Gastric Intestinal Metaplasia by the Genta Stain
- Comparison of Intestinal Metaplasia and Serum Pepsinogen Levels between Helicobacter pylori-Infected Duodenal Ulcer and Chronic Gastritis
- How Could We Improve Atrophic Gastritis and Intestinal Metaplasia?
- Natural Course of Atrophic Gastritis and Intestinal Metaplasia
- Reversibility of Atrophic Gastritis and Intestinal Metaplasia by Eradication of Helicobacter pylori