Lab Anim Res.
2017 Sep;33(3):256-263. 10.5625/lar.2017.33.3.256.
In vivo validation of metastasis-regulating microRNA-766 in human triple-negative breast cancer cells
- Affiliations
-
- 1Laboratory of Immunology/Cancer Biology, Department of Biomedical Sciences, Seoul National University College of Medicine, 103 Daehakro, Jongno-gu, Seoul, Korea. dlee5522@snu.ac.kr
- 2Cancer Research Institute, Interdisciplinary Program of Tumor Biology, Seoul National University College of Medicine, Seoul, Korea.
- 3Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2391100
- DOI: http://doi.org/10.5625/lar.2017.33.3.256
Abstract
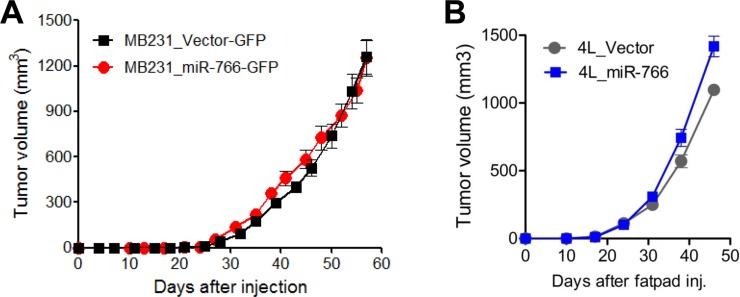
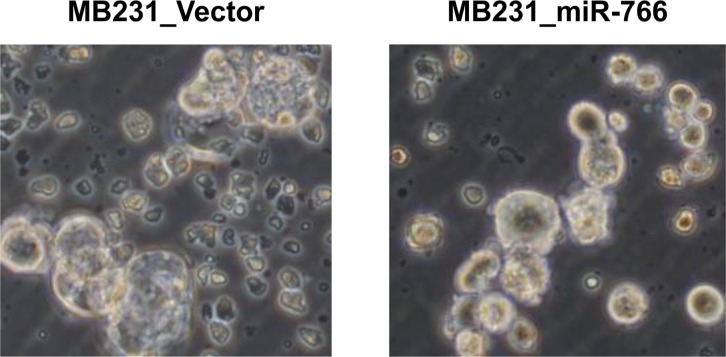
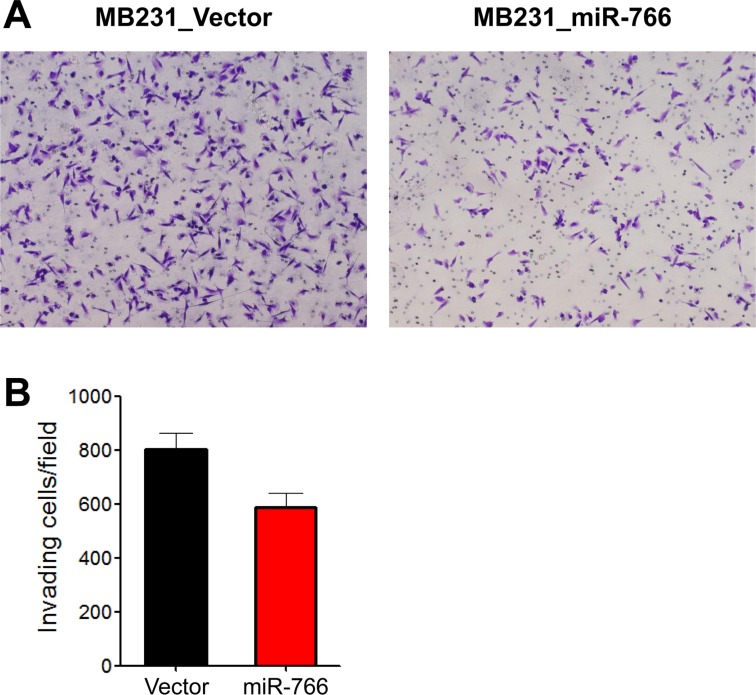
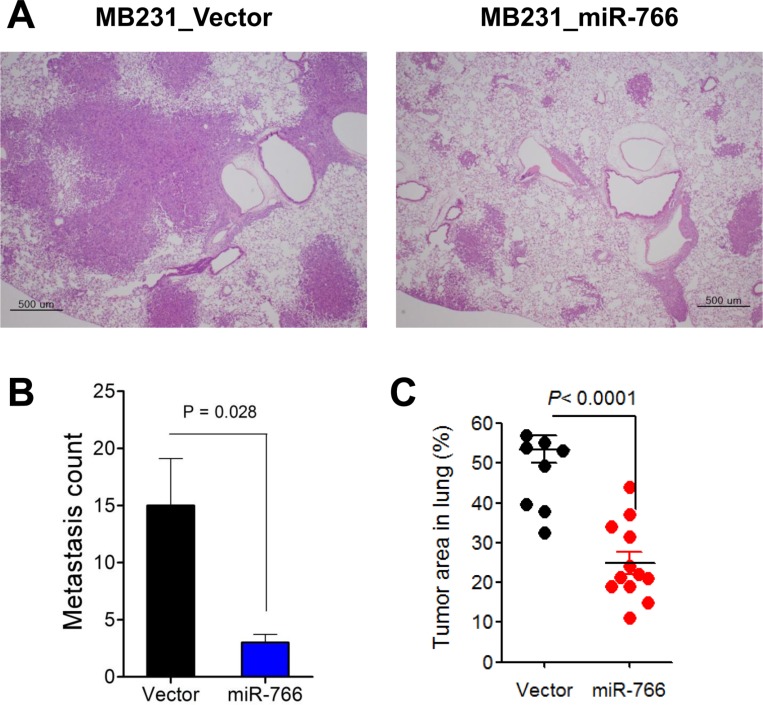
- Breast cancer is the second most common cancer and the most frequent cancer in women worldwide. Recent improvements in early detection and effective adjuvant chemotherapies have improved the survival of breast cancer patients. Even with initial disease remission, one-third of all breast cancer patients will relapse with distant metastasis. Breast cancer metastasis is largely an incurable disease and the main cause of death among breast cancer patients. Cancer metastasis is comprised of complex processes that are usually not controllable by intervention of a single molecular target. As a single microRNA (miRNA) can affect the aggressiveness of breast cancer cells by concurrently modulating multiple pathway effectors, a metastasis-regulating miRNA would represent a good disease target candidate. In this study, we evaluated the functional capacity of a newly defined human metastasis-related miRNA, miR-766, which was previously identified by comparing a patient-derived xenograft primary tumor model and a metastasis model. Compared to vector-transfected control cells, miR-766-overexpressed triple-negative breast cancer cells exhibited similar primary tumor growth in the orthotopic xenograft model. In contrast, tumor sphere formation and Matrigel invasion were significantly decreased in miR-766-overexpressed breast cancer cells compared with control cancer cells. In addition, lung metastasis was dramatically reduced in miR-766-overexpressed breast cancer cells compared with control cells. Thus, miR-766 affected the distant metastasis process to a greater extent than cancer cell proliferation and primary tumor growth, and may represent a future therapeutic target to effectively control fatal breast cancer metastasis.
MeSH Terms
Figure
Reference
-
1. American Cancer Society. Breast Cancer Facts & Figures 2011-2012. http://www.cancer.org/Research/CancerFactsFigures/BreastCancerFactsFigures/breast-cancer-facts-and-figures-2011-2012.2. Polyak K. Breast cancer: origins and evolution. J Clin Invest. 2007; 117(11):3155–3163. PMID: 17975657.
Article3. Hornberger J, Alvarado MD, Rebecca C, Gutierrez HR, Yu TM, Gradishar WJ. Clinical validity/utility, change in practice patterns, and economic implications of risk stratifiers to predict outcomes for early-stage breast cancer: a systematic review. J Natl Cancer Inst. 2012; 104(14):1068–1079. PMID: 22767204.
Article4. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365(9472):1687–1717. PMID: 15894097.5. El Saghir NS, Tfayli A, Hatoum HA, Nachef Z, Dinh P, Awada A. Treatment of metastatic breast cancer: state-of-the-art, subtypes and perspectives. Crit Rev Oncol Hematol. 2011; 80(3):433–449. PMID: 21330148.
Article6. Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012; 11(6):479–497. PMID: 22653217.
Article7. Oh K, Ko E, Kim HS, Park AK, Moon HG, Noh DY, Lee DS. Transglutaminase 2 facilitates the distant hematogenous metastasis of breast cancer by modulating interleukin-6 in cancer cells. Breast Cancer Res. 2011; 13(5):R96. PMID: 21967801.
Article8. Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, Lee DS. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res. 2013; 15(5):R79. PMID: 24021059.
Article9. Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010; 11(4):252–263. PMID: 20216554.
Article10. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136(2):215–233. PMID: 19167326.
Article11. Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015; 16(7):421–433. PMID: 26077373.
Article12. Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs--the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009; 9(4):293–302. PMID: 19262572.13. Moon HG, Oh K, Lee J, Lee M, Kim JY, Yoo TK, Seo MW, Park AK, Ryu HS, Jung EJ, Kim N, Jeong S, Han W, Lee DS, Noh DY. Prognostic and functional importance of the engraftment-associated genes in the patient-derived xenograft models of triple-negative breast cancers. Breast Cancer Res Treat. 2015; 154(1):13–22. PMID: 26438141.
Article14. Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002; 100(9):3175–3182. PMID: 12384415.15. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lønning PE, Børresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000; 406(6797):747–752. PMID: 10963602.
Article16. Ahmad A. Pathways to breast cancer recurrence. ISRN Oncol. 2013; 2013:290568. PMID: 23533807.
Article17. Santos R, Ursu O, Gaulton A, Bento AP, Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI, Overington JP. A comprehensive map of molecular drug targets. Nat Rev Drug Discov. 2017; 16(1):19–34. PMID: 27910877.18. Mimeault M, Batra SK. Altered gene products involved in the malignant reprogramming of cancer stem/progenitor cells and multitargeted therapies. Mol Aspects Med. 2014; 39:3–32. PMID: 23994756.
Article19. Gandellini P, Doldi V, Zaffaroni N. microRNAs as players and signals in the metastatic cascade: Implications for the development of novel anti-metastatic therapies. Semin Cancer Biol. 2017; 44:132–140. PMID: 28344166.
Article20. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017; 16(3):203–222. PMID: 28209991.
Article21. Teoh SL, Das S. The Role of MicroRNAs in Diagnosis, Prognosis, Metastasis and Resistant Cases in Breast Cancer. Curr Pharm Des. 2017; 23(12):1845–1859. PMID: 28231756.
Article22. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016; 16(9):582–598. PMID: 27550820.
Article23. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017; 14(7):399–416. PMID: 28117416.
Article24. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012; 12(4):253–268. PMID: 22437938.
Article25. Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017; 17(5):302–317. PMID: 28303905.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long Noncoding-RNA Component of Mitochondrial RNA Processing Endoribonuclease Promotes Carcinogenesis in Triple-Negative Breast Cancer Cells via the Competing Endogenous RNA Mechanism
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- Hsa-miRNA-143-3p Reverses Multidrug Resistance of Triple-Negative Breast Cancer by Inhibiting the Expression of Its Target Protein Cytokine-Induced Apoptosis Inhibitor 1 In Vivo
- Comment to “Patients with Concordant Triple-Negative Phenotype between Primary Breast Cancers and Corresponding Metastases Have Poor Prognosisâ€
- Fear of Cancer Recurrence and Unmet Needs in Triple Negative Breast Cancer Survivors