J Dent Anesth Pain Med.
2017 Sep;17(3):191-198. 10.17245/jdapm.2017.17.3.191.
Effects of nerve cells and adhesion molecules on nerve conduit for peripheral nerve regeneration
- Affiliations
-
- 1Division of Oral and Maxillofacial Surgery, Department of Dentistry, College of Medicine, Hanyang University, Seoul, Korea. fastchang@hanyang.ac.kr
- 2Department of Periodontics, University of Pennsylvania School of Dental Medicine, Philadelphia, PA, USA.
- KMID: 2390803
- DOI: http://doi.org/10.17245/jdapm.2017.17.3.191
Abstract
- BACKGROUND
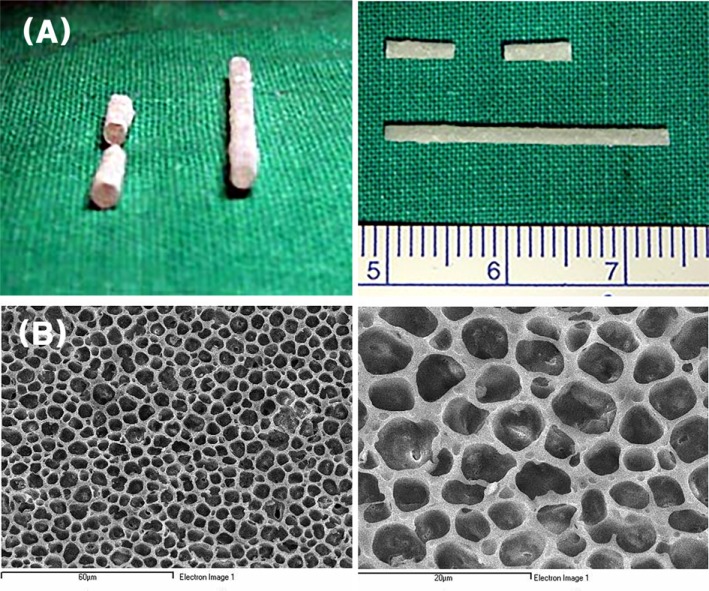
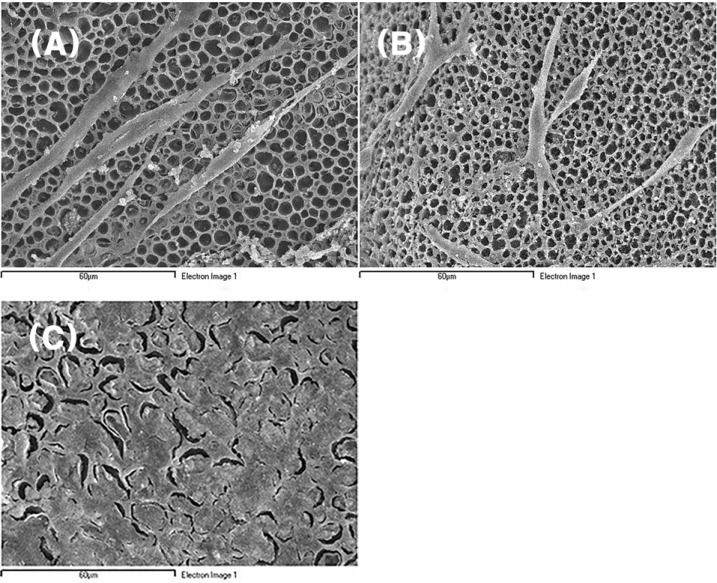
For peripheral nerve regeneration, recent attentions have been paid to the nerve conduits made by tissue-engineering technique. Three major elements of tissue-engineering are cells, molecules, and scaffolds.
METHODS
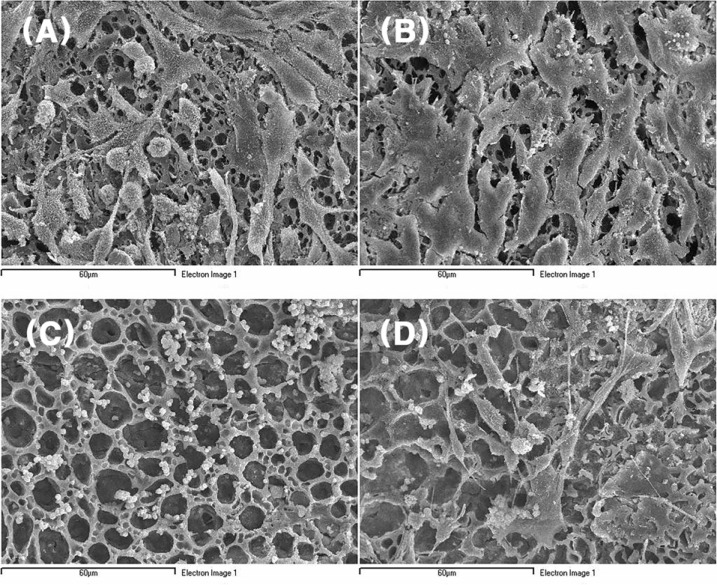
In this study, the attachments of nerve cells, including Schwann cells, on the nerve conduit and the effects of both growth factor and adhesion molecule on these attachments were investigated.
RESULTS
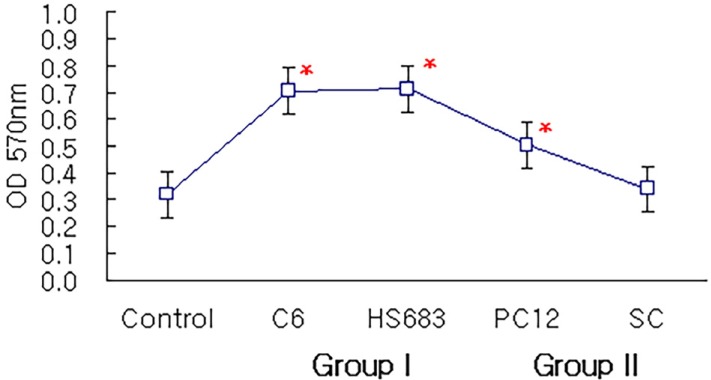
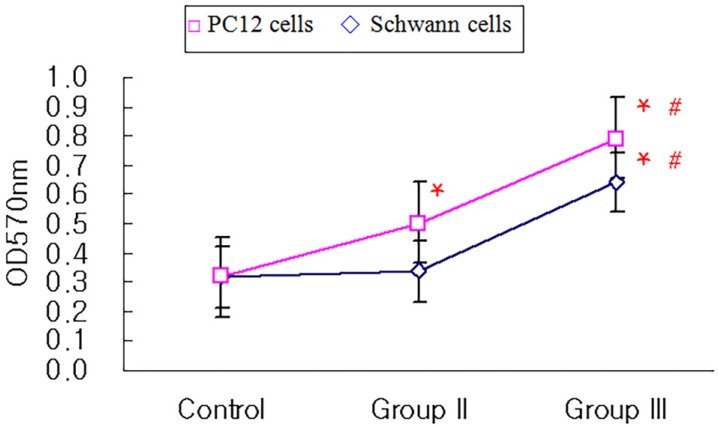
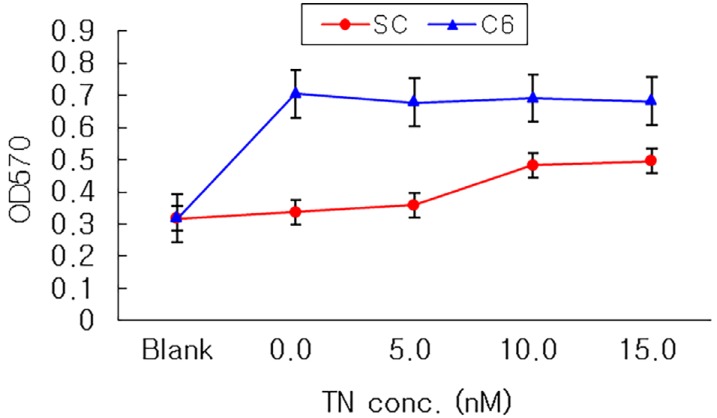
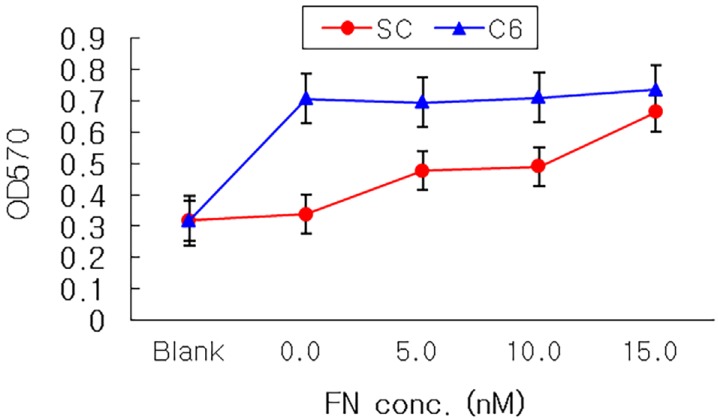
The attachment of rapidly-proliferating cells, C6 cells and HS683 cells, on nerve conduit was better than that of slowly-proliferating cells, PC12 cells and Schwann cells, however, the treatment of nerve growth factor improved the attachment of slowly-proliferating cells. In addition, the attachment of Schwann cells on nerve conduit coated with fibronectin was as good as that of Schwann cells treated with glial cell line-derived neurotrophic factor (GDNF).
CONCLUSIONS
Growth factor changes nerve cell morphology and affects cell cycle time. And nerve growth factor or fibronectin treatment is indispensable for Schwann cell to be used for implantation in artificial nerve conduits.
MeSH Terms
Figure
Reference
-
1. Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007; 23:511–520. PMID: 18189213.
Article2. Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011; 39:81–124. PMID: 21488817.
Article3. Colen KL, Choi M, Chiu DT. Nerve grafts and conduits. Plast Reconstr Surg. 2009; 124(6 suppl):e386–e394. PMID: 19952706.
Article4. Hadlock T, Elisseeff J, Langer R, Vacanti J, Cheney M. A tissue-engineered conduit for peripheral nerve repair. Arch Otolaryngol Head Neck Surg. 1998; 124:1081–1086. PMID: 9776185.
Article5. Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, et al. Bioactive poly(L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials. 2002; 23:841–848. PMID: 11774850.
Article6. Takata T, Wang HL, Miyauchi M. Migration of osteoblastic cells on various guided bone regeneration membranes. Clin Oral Implants Res. 2001; 12:332–338. PMID: 11488862.
Article7. Fine EG, Decosterd I, Papaloizos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002; 15:589–601. PMID: 11886440.
Article8. Kotwal A, Schmidt CE. Electrical stimulation alters protein adsorption and nerve cell interactions with electrically conducting biomaterials. Biomaterials. 2001; 22:1055–1064. PMID: 11352099.
Article9. Shen ZL, Lassner F, Becker M, Walter GF, Bader A, Berger A. Viability of cultured nerve grafts: An assessment of proliferation of Schwann cells and fibroblasts. Microsurgery. 1999; 19:356–363. PMID: 10594909.
Article10. Jia Z, Shen D, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001; 333:1–6. PMID: 11423105.
Article11. Bryan DJ, Holway AH, Wang KK, Silva AE, Trantolo DJ, Wise D, et al. Influence of glial growth factor and Schwann cells in a bioresorbable guidance channel on peripheral nerve regeneration. Tissue Eng. 2000; 6:129–138. PMID: 10941208.
Article12. Araki T, Nagarajan R, Milbrandt J. Identification of genes induced in peripheral nerve after injury. Expression profiling and novel gene discovery. J Biol Chem. 2001; 276:34131–34141. PMID: 11427537.13. Copertino DW, Edelman GM, Jones FS. Multiple promoter elements differentially regulate the expression of the mouse tenascin gene. Proc Natl Acad Sci U S A. 1997; 94:1846–1851. PMID: 9050867.
Article14. Pellen-Mussi P, Fravalo P, Guigand M, Bonnaure-Mallet M. Evaluation of cellular proliferation on collagenous membranes. J Biomed Mater Res. 1997; 36:331–336. PMID: 9260104.
Article15. Chernousov MA, Carey DJ. Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol. 2000; 15:593–601. PMID: 10809381.16. Probstmeier R, Nellen J, Gloor S, Wernig A, Pesheva P. Tenascin-R is expressed by Schwann cells in the peripheral nervous system. J Neurosci Res. 2001; 64:70–78. PMID: 11276053.
Article17. Jakovcevski I, Miljkovic D, Schachner M, Andjus PR. Tenascins and inflammation in disorders of the nervous system. Amino Acids. 2013; 44:1115–1127. PMID: 23269478.
Article18. Ahmed Z, Brown RA. Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil Cytoskeleton. 1999; 42:331–343. PMID: 10223638.
Article19. Chen YS, Hsieh CL, Tsai CC, Chen TH, Cheng WC, Hu CL, et al. Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials. 2000; 21:1541–1547. PMID: 10885726.
Article20. Varon S, Adler R. Nerve growth factors and control of nerve growth. Curr Top Dev Biol. 1980; 16:207–252. PMID: 6258863.21. Simain-Sato F, Lahmouzi J, Kalykakis GK, Heinen E, Defresne MP, De Pauw MC, et al. Culture of gingival fibroblasts on bioabsorbable regenerative materials in vitro. J Periodontol. 1999; 70:1234–1239. PMID: 10534079.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of several nerve conduit on peripheral nerve regeneration in rabbits
- Peripheral nerve regeneration using a three-dimensionally cultured schwann cell conduit
- Effects of Fresh and Degenerated Autogenous Nerve Graft in Segmental Defect of Sciatic Nerve of Rabbit
- Peripheral Nerve Regeneration Using a Nerve Conduit with Olfactory Ensheathing Cells in a Rat Model
- Development of microporous calcium phosphate coated nerve conduit for peripheral nerve repair