J Korean Ophthalmol Soc.
2017 Sep;58(9):1050-1057. 10.3341/jkos.2017.58.9.1050.
Clinical Analysis of Newly Diagnosed Diabetes Mellitus Patients by Abnormal Fundus Examination
- Affiliations
-
- 1Department of Ophthalmology, Nowon Eulji Medical Center, Eulji University School of Medicine, Seoul, Korea. pjs4106@eulji.ac.kr
- KMID: 2390068
- DOI: http://doi.org/10.3341/jkos.2017.58.9.1050
Abstract
- PURPOSE
To investigate the clinical analysis of newly diagnosed diabetes mellitus (NDM) patients with abnormal fundus examination at the first visit.
METHODS
This retrospective study utilized the first visit medical records of 15 patients (30 eyes) who were diagnosed with NDM from February 2011 to October 2016.
RESULTS
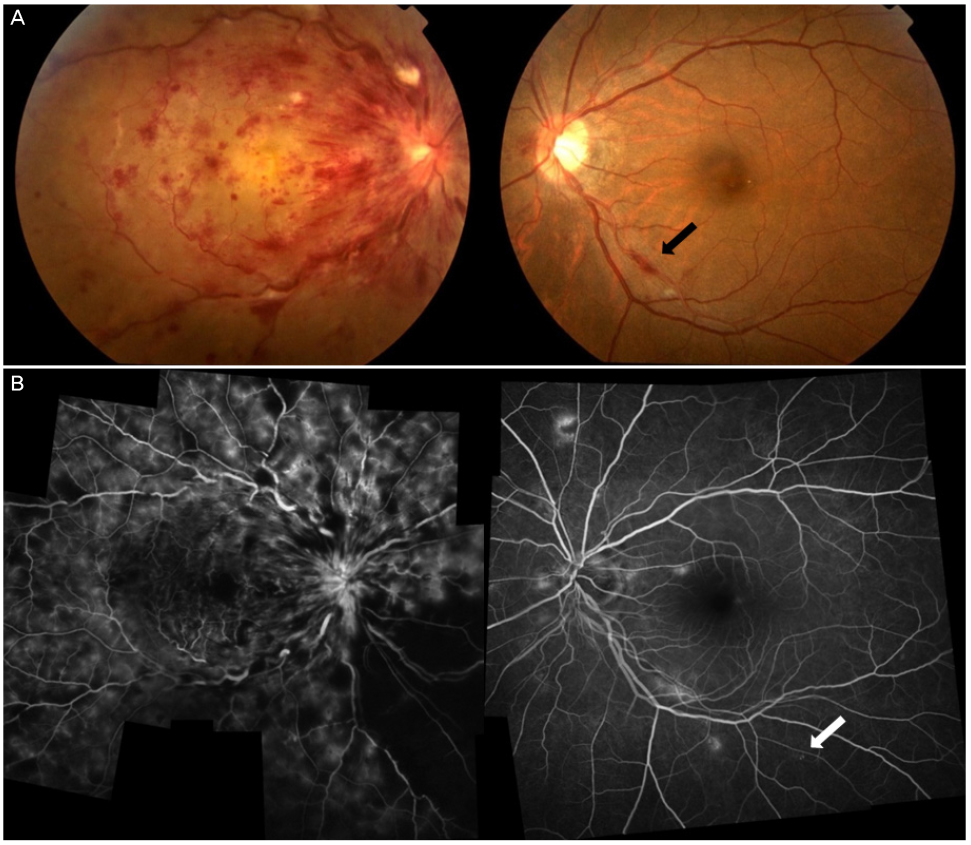
Patients were divided into 3 groups: 1) diabetic retinopathy group including proliferative diabetic retinopathy (PDR) (3) and severe non-proliferative diabetic retinopathy (NPDR) (1); 2) retinal vascular disease group including central retinal vein occlusion (CRVO) (1), branch retinal vein occlusion (1), vitreous hemorrhage with CRVO (1) and macular edema (1); and 3) other retinal disease group including vitreous hemorrhage due to choroidal neovascular rupture (1), exudative age-related macular degeneration (3), central serous chorioretinopathy (2), and macular hole (1). All 3 PDR patients had latent autoimmune diabetes in adults (type 1.5 diabetes). The remaining 12 patients had type 2 diabetes. Three patients showed mild NPDR in the opposite eye and the other 9 patients did not have diabetic retinopathy in the opposite eye. Onset age, HbA1C and proteinuria were significantly different between the diabetic retinopathy group and the other retinal disease group (p = 0.006, p = 0.012 and p = 0.006, Mann-Whitney test).
CONCLUSIONS
In patients with various retinal diseases, early detection of NDM could be achieved by performing fundoscopic imaging and systemic examination as well as basic ophthalmologic examination. In addition, patients with diabetic retinopathy should be treated promptly through ophthalmology and internal medicine consultation. For the retinal vascular disease and other retinal disease groups, not only treatment for ophthalmic diseases, but also education about diabetes treatment are important.
Keyword
MeSH Terms
-
Adult
Age of Onset
Central Serous Chorioretinopathy
Choroid
Diabetes Mellitus*
Diabetes Mellitus, Type 1
Diabetic Retinopathy
Education
Humans
Internal Medicine
Macular Degeneration
Macular Edema
Medical Records
Ophthalmology
Proteinuria
Retinal Diseases
Retinal Perforations
Retinal Vein
Retinal Vein Occlusion
Retinaldehyde
Retrospective Studies
Rupture
Vascular Diseases
Vitreous Hemorrhage
Retinaldehyde
Figure
Reference
-
1. Drivsholm T, de Fine Olivarius N, Nielsen AB, Siersma V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia. 2005; 48:210–214.2. Colagiuri S, Cull CA, Holman RR. UKPDS Group. Are lower fasting plasma glucose levels at diagnosis of type 2 diabetes associated with improved outcomes?: U.K. prospective diabetes study 61. Diabetes Care. 2002; 25:1410–1417.3. Wahab S, Mahmood N, Shaikh Z, Kazmi WH. Frequency of retinopathy in newly diagnosed type 2 diabetes patients. J Pak Med Assoc. 2008; 58:557–561.4. Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992; 15:815–819.5. Abdollahi A, Malekmadani MH, Mansoori MR, et al. Prevalence of diabetic retinopathy in patients with newly diagnosed type II diabetes mellitus. Acta Med Iran. 2006; 44:415–419.6. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35:556–564.7. Tapp RJ, Shaw JE, Harper CA, et al. The prevalence of and factors associated with diabetic retinopathy in the Australian population. Diabetes Care. 2003; 26:1731–1737.8. Klein R, Klein BE, Moss SE, Linton KL. The Beaver Dam Eye Study: retinopathy in adults with newly discovered and previously diagnosed diabetes mellitus. Ophthalmology. 1992; 99:58–62.9. Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000; 98:133–141. discussion 141-3.10. Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009; 116:1928–1936.11. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. 2004; 27:Suppl 1. S11–S14.12. Tuomi T, Santoro N, Caprio S, et al. The many faces of diabetes: a disease with increasing heterogeneity. Lancet. 2014; 383:1084–1094.13. Juneja R, Hirsch IB, Naik RG, et al. Islet cell antibodies and glutamic acid decarboxylase antibodies, but not the clinical phenotype, help to identify type 1(1/2) diabetes in patients presenting with type 2 diabetes. Metabolism. 2001; 50:1008–1013.14. Roh MO, Jung CH, Kim BY, et al. The prevalence and characteristics of latent autoimmune diabetes in adults (LADA) and its relation with chronic complications in a clinical department of a university hospital in Korea. Acta Diabetol. 2013; 50:129–134.15. Stenström G, Gottsäter A, Bakhtadze E, et al. Latent autoimmune diabetes in adults: definition, prevalence, beta-cell function, and treatment. Diabetes. 2005; 54:Suppl 2. S68–S72.16. Arikan E, Sabuncu T, Ozer EM, Hatemi H. The clinical characteristics of latent autoimmune diabetes in adults and its relation with chronic complications in metabolically poor controlled Turkish patients with Type 2 diabetes mellitus. J Diabetes Complications. 2005; 19:254–258.17. Myhill P, Davis WA, Bruce DG, et al. Chronic complications and mortality in community-based patients with latent autoimmune diabetes in adults: the Fremantle Diabetes Study. Diabet Med. 2008; 25:1245–1250.18. Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004; 27:Suppl 1. S84–S87.19. Chistiakov DA. Diabetic retinopathy: pathogenic mechanisms and current treatments. Diabetes Metab Syndr. 2011; 5:165–172.20. Harding SP, Broadbent DM, Neoh C, et al. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ. 1995; 311:1131–1135.21. Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case–control study. Ophthalmology. 2004; 111:244–249.22. Clemons TE, Milton RC, Klein R, et al. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology. 2005; 112:533–539.23. Evans JR, Schwartz SD, McHugh JD, et al. Systemic risk factors for idiopathic macular holes: a case-control study. Eye (Lond). 1998; 12(Pt 2):256–259.24. Mogensen CE, Chachati A, Christensen CK, et al. Microalbuminuria: an early marker of renal involvement in diabetes. Uremia Invest. 1985-1986; 9:85–95.25. Cruickshanks KJ, Ritter LL, Klein R, Moss SE. The association of microalbuminuria with diabetic retinopathy. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Ophthalmology. 1993; 100:862–867.26. Matthews DR, Stratton IM, Aldington SJ, et al. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004; 122:1631–1640.27. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998; 317:703–713.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fundus Examination Rate in Diabetics and the Public Health Factors Associated With Fundus Examination Rate
- A Case of Newly Diagnosed Type 2 Diabetes Mellitus with Daily Headaches
- The Initial Fundus Examination and Severity of Diabetic Retinopathy in Diabetic Patients Diagnosed Over 30 Years of Age
- Main Reasons for and Associated Factors of the First Fundus Examination in Diabetic Patients
- Relationship between Clinical Features of Diabetic Retinopathy and Systemic Factors in Patients with Newly Diagnosed Type II Diabetes Mellitus