J Breast Cancer.
2017 Jun;20(2):142-149. 10.4048/jbc.2017.20.2.142.
CD49f Can Act as a Biomarker for Local or Distant Recurrence in Breast Cancer
- Affiliations
-
- 1Laboratory of Pathology, State Key Laboratory of Biotherapy, National Collaborative Innovation Center for Biotherapy, Chengdu, China.
- 2Cancer Center, State Key Laboratory of Biotherapy, National Collaborative Innovation Center for Biotherapy, Chengdu, China.
- 3Laboratory of Molecular Diagnosis of Cancer, State Key Laboratory of Biotherapy, National Collaborative Innovation Center for Biotherapy, Chengdu, China.
- 4Department of Pathology, West China Hospital, Sichuan University, Chengdu, China. molecularpathology@hotmail.com
- KMID: 2389751
- DOI: http://doi.org/10.4048/jbc.2017.20.2.142
Abstract
- PURPOSE
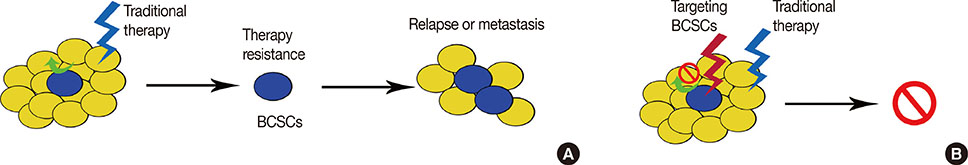
Metastasis and local recurrence are the primary causes of treatment failure and patient death in breast cancer. The aim of this study was to validate a metastasis- and local recurrenceassociated biomarker for prognostic evaluation and planning treatment strategies.
METHODS
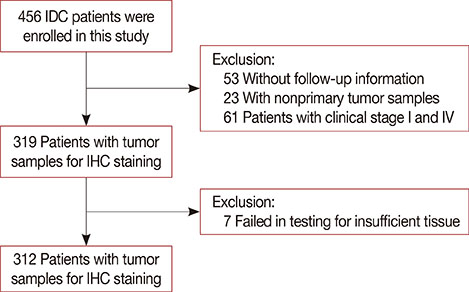
Formalin-fixed, paraffin-embedded tissues from a cohort of 312 patients (all stage II and III) were used. The prevalence of CD49f⺠cells in the patients' tumors was analyzed and correlated with clinical characteristics to determine its prognostic and clinical implications.
RESULTS
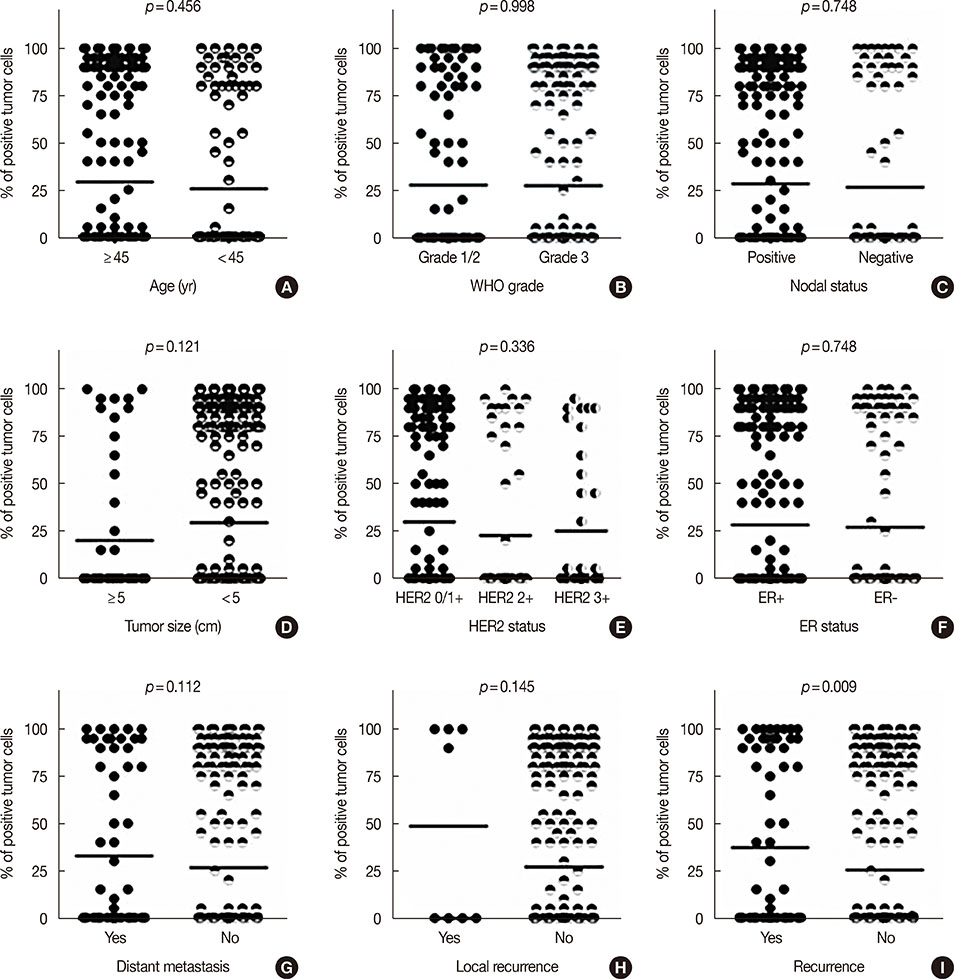
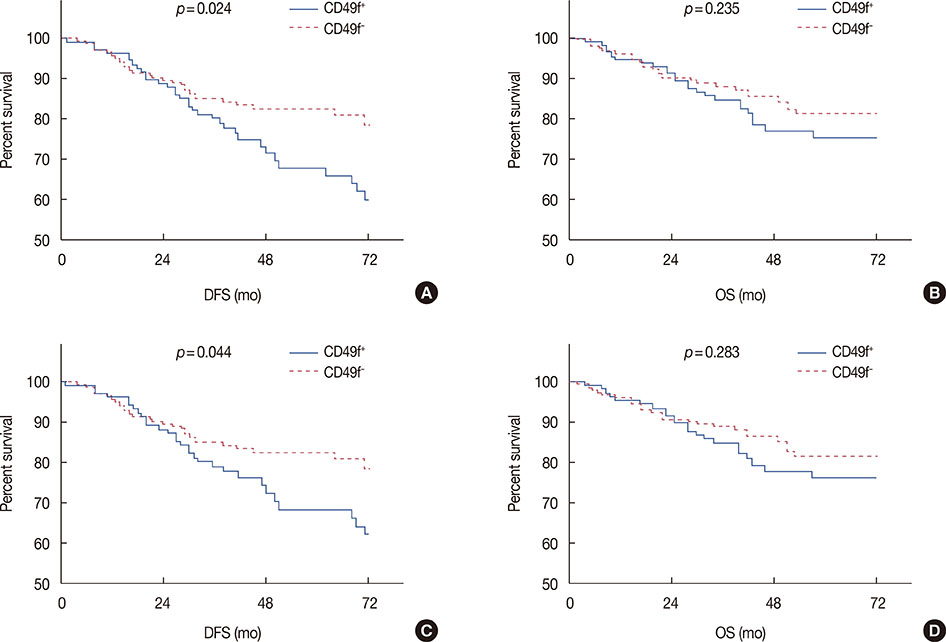
CD49f⺠tumor cells were found in a minority of tumors, with 62.8% of the samples showing not a single cell of this subtype. In the clinical characteristics analysis, which were performed with t-tests, CD49f⺠tumors were not associated with age, tumor size, World Health Organization grade, nodal status, human epidermal growth factor receptor 2 status, progesterone receptor status, or estrogen receptor status, although they were significantly associated with disease recurrence (distant metastasis or/and local recurrence). Univariate survival analysis using the Kaplan-Meier method showed that CD49f⺠tumors were associated with markedly decreased disease-free survival (DFS); the same result was found using multivariate Cox analysis, even when only chemotherapy-treated patients were analyzed.
CONCLUSION
Our results indicated that breast tumors with CD49f⺠cancer cells are associated with an increased risk for disease recurrence after initial surgery with poor clinical outcomes (decreased DFS). Therefore, as it requires testing for only one additional protein, adding CD49f testing to conventional surgical pathology is a strategy that has great potential for prognostic and treatment-guidance purposes.
MeSH Terms
-
Breast Neoplasms*
Breast*
Cohort Studies
Disease-Free Survival
Estrogens
Humans
Integrin alpha6
Methods
Neoplasm Metastasis
Neoplastic Stem Cells
Pathology, Surgical
Prevalence
Prognosis
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Recurrence*
Treatment Failure
World Health Organization
Estrogens
Integrin alpha6
Receptor, Epidermal Growth Factor
Receptors, Progesterone
Figure
Reference
-
1. American Cancer Society. Cancer Treatment & Survivorship Fact & Figures 2012-2013. Atlanta: American Cancer Society;2012.2. Ye F, Qiu Y, Li L, Yang L, Cheng F, Zhang H, et al. The presence of EpCAM(-)/CD49f(+) cells in breast cancer is associated with a poor clinical outcome. J Breast Cancer. 2015; 18:242–248.
Article3. Qiu Y, Pu T, Li L, Cheng F, Lu C, Sun L, et al. The expression of aldehyde dehydrogenase family in breast cancer. J Breast Cancer. 2014; 17:54–60.
Article4. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004; 351:2817–2826.
Article5. Saghatchian M, Mook S, Pruneri G, Viale G, Glas AM, Guerin S, et al. Additional prognostic value of the 70-gene signature (MammaPrint(®)) among breast cancer patients with 4-9 positive lymph nodes. Breast. 2013; 22:682–690.
Article6. Sapino A, Roepman P, Linn SC, Snel MH, Delahaye LJ, van den Akker J, et al. MammaPrint molecular diagnostics on formalin-fixed, paraffin-embedded tissue. J Mol Diagn. 2014; 16:190–197.
Article7. Slodkowska EA, Ross JS. MammaPrint 70-gene signature: another milestone in personalized medical care for breast cancer patients. Expert Rev Mol Diagn. 2009; 9:417–422.
Article8. Bellocq JP, Luporsi E, Barrière J, Bonastre J, Chetritt J, Le Corroller AG, et al. uPA/PAI-1, Oncotype DX™, MammaPrint(®): prognosis and predictive values for clinical utility in breast cancer management. Ann Pathol. 2014; 34:349–351.
Article9. Ahmad S, Gupta S, Kumar R, Varshney GC, Raghava GP. Herceptin resistance database for understanding mechanism of resistance in breast cancer patients. Sci Rep. 2014; 4:4483.
Article10. Martin-Castillo B, Oliveras-Ferraros C, Vazquez-Martin A, Cufí S, Moreno JM, Corominas-Faja B, et al. Basal/HER2 breast carcinomas: integrating molecular taxonomy with cancer stem cell dynamics to predict primary resistance to trastuzumab (Herceptin). Cell Cycle. 2013; 12:225–245.11. Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005; 11:1154–1159.12. Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009; 15:907–913.
Article13. Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010; 28:2784–2795.
Article14. Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008; 10:R53.15. Sun B, Zhang S, Zhang D, Li Y, Zhao X, Luo Y, et al. Identification of metastasis-related proteins and their clinical relevance to triple-negative human breast cancer. Clin Cancer Res. 2008; 14:7050–7059.
Article16. Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007; 7:737–749.
Article17. Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003; 3:453–458.
Article18. Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011; 147:992–1009.
Article19. Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014; 158:1110–1122.
Article20. Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011; 178:989–996.
Article21. Pfefferle AD, Spike BT, Wahl GM, Perou CM. Luminal progenitor and fetal mammary stem cell expression features predict breast tumor response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2015; 149:425–437.
Article22. Yang Y, Otte A, Hass R. Human mesenchymal stroma/stem cells exchange membrane proteins and alter functionality during interaction with different tumor cell lines. Stem Cells Dev. 2015; 24:1205–1222.
Article23. Zheng Z, Shao N, Weng H, Li W, Zhang J, Zhang L, et al. Correlation between epidermal growth factor receptor and tumor stem cell markers CD44/CD24 and their relationship with prognosis in breast invasive ductal carcinoma. Med Oncol. 2015; 32:275.
Article24. Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, et al. Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell. 2014; 14:357–369.
Article25. Huang Y, Yu P, Li W, Ren G, Roberts AI, Cao W, et al. p53 regulates mesenchymal stem cell-mediated tumor suppression in a tumor microenvironment through immune modulation. Oncogene. 2014; 33:3830–3838.
Article26. Badve S, Nakshatri H. Breast-cancer stem cells-beyond semantics. Lancet Oncol. 2012; 13:e43–e48.
Article27. Hwang R, Varner J. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004; 18:991–1006.
Article28. Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008; 8:604–617.
Article29. Vassilopoulos A, Chisholm C, Lahusen T, Zheng H, Deng CX. A critical role of CD29 and CD49f in mediating metastasis for cancer-initiating cells isolated from a Brca1-associated mouse model of breast cancer. Oncogene. 2014; 33:5477–5482.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Presence of EpCAM-/CD49f+ Cells in Breast Cancer Is Associated with a Poor Clinical Outcome
- The Impact of Local and Regional Recurrence on Distant Metastasis and Survival in Patients Treated with Breast Conservation Therapy
- Small bowel obstruction from distant metastasis of primary breast cancer: a case report
- Clinicopathologic Characteristics and Local Recurrence after Skin-sparing Mastectomy with Immediate Reconstruction
- Lumpectomy with Axillary Dissection for Breast Cancer