J Korean Ophthalmol Soc.
2017 Jul;58(7):879-884. 10.3341/jkos.2017.58.7.879.
Tube Erosion with Scleral Melting after Ahmed Valve Implantation Using a Synthetic Dural Substitute
- Affiliations
-
- 1Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, National Health Insurance Service Ilsan Hospital, Goyang, Korea. unij413@naver.com
- KMID: 2387166
- DOI: http://doi.org/10.3341/jkos.2017.58.7.879
Abstract
- PURPOSE
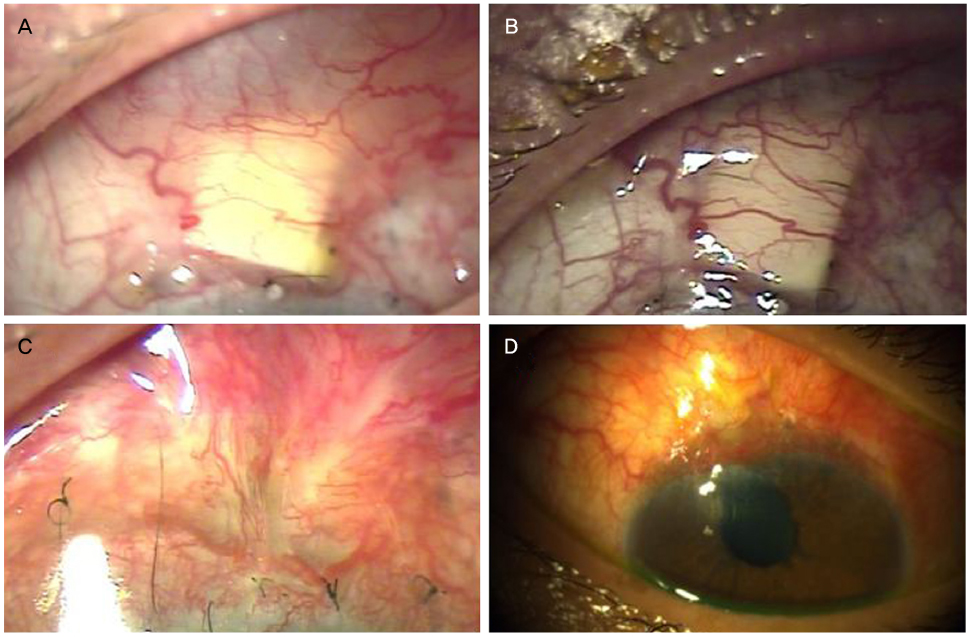
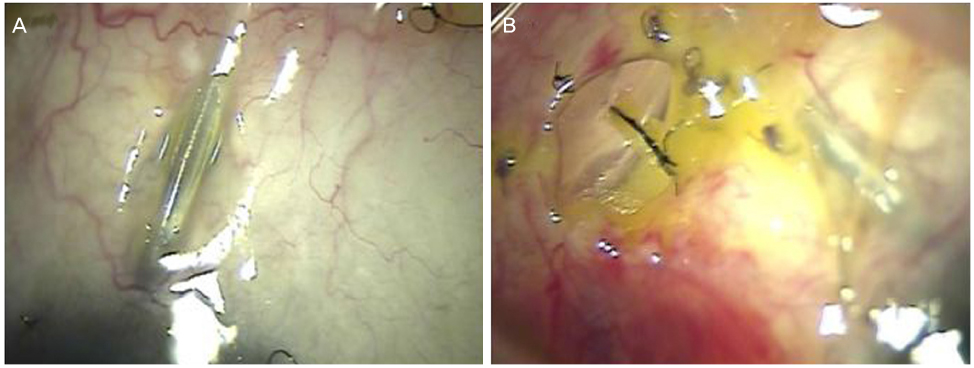
The objective of this case report was to present tube erosion of Ahmed valve implantation using a synthetic dura substitute (Neuro-Patch®, B. Braun, Boulogne, France).
CASE SUMMARY
Tube erosion was caused by dissolution of the conjunctiva and partial-thickness scleral tunnel in 5 patients who received Ahmed valve implantation using a synthetic dura substitute for glaucoma treatment 2 to 4 months after the operation. Furthermore, the patients required re-operation for preventing secondary complications such as endophthalmitis.
CONCLUSIONS
This case series using a synthetic dura substitute in Ahmed valve implantation demonstrated the risk of tube erosion with scleral tunnel melting and following secondary complications even with a partial-thickness scleral tunnel method.
Figure
Reference
-
1. Lim K, Allan BD, Lloyd AW, et al. Glaucoma drainage devices; past, present, and future. Br J Ophthalmol. 1998; 82:1083–1089.2. Rosenberg LF, Krupin T. Implants in glaucoma surgery. The Glaucomas. 1996; 3:1783–1807.3. Smith MF, Doyle JW, Ticrney JW Jr. A comparison of glaucoma drainage implant tube coverage. J Glaucoma. 2002; 11:143–147.4. Rosentreter A, Mellein AC, Konen WW, Dietlein TS. Capsule excision and Ologen implantation for revision after glaucoma drainage device surgery. Graefes Arch Clin Exp Ophthalmol. 2010; 248:1319–1324.5. Malliti M, Page P, Gury C, et al. Comparison of deep wound infection rates using a synthetic dural substitute (neuro-patch) or pericranium graft for dural closure: a clinical review of 1 year. Neurosurgery. 2004; 54:599–603. discussion 603-4.6. Dubey S, Sharma V, Agrawal A, et al. Safety and efficacy of Ahmed glaucoma valve implantation in refractory glaucomas in Northern Indian eyes. Saudi J Ophthalmol. 2015; 29:103–108.7. Kim SW, Kim YH, Yun IS, Ahn JH. Surgical treatment for tube erosion after Ahmed valve implantation. J Korean Ophthalmol Soc. 2016; 57:453–460.8. Huang MC, Netland PA, Coleman AL, et al. Intermediate-term clinical experience with the Ahmed Glaucoma Valve implant. Am J Ophthalmol. 1999; 127:27–33.9. Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003; 136:464–470.10. Heuer DK, Budenz D, Coleman A. Aqueous shunt tube erosion. J Glaucoma. 2001; 10:493–496.11. Francis BA, DiLoreto DA, Chong LP, Rao N. Late-onset bacterial endophthalmitis following glaucoma drainage implantation. Ophthalmic Surg Lasers Imaging. 2003; 34:128–130.12. Melamed S, Fiore PM. Molteno implant surgery in refractory glaucoma. Surv Ophthalmol. 1990; 34:441–448.13. Trubnik V, Zangalli C, Moster MR, et al. Evaluation of risk factors for glaucoma drainage device-related erosions: a retrospective case-control study. J Glaucoma. 2015; 24:498–502.14. Rosentreter A, Schild AM, Dinslage S, Dietlein TS. Biodegradable implant for tissue repair after glaucoma drainage device surgery. J Glaucoma. 2012; 21:76–78.15. Gudmundsson G, Søgaard I. Complications to the use of vicryl-collagen dural substitute. Acta Neurochir (Wien). 1995; 132:145–147.16. Raul JS, Godard J, Arbez-Gindre F, Czorny A. Use of polyester urethane (Neuro-Patch) as a dural substitute. Prospective study of 70 cases. Neurochirurgie. 2003; 49(2-3 Pt 1):83–89.17. Cardwell RD, Kluge JA, Thayer PS, et al. Static and cyclic mechanical loading of mesenchymal stem cells on elastomeric, electrospun polyurethane meshes. J Biomech Eng. 2015; 137:DOI: 10.1115/1.4030404. Epub 2015 Jun 3.18. Zanetta M, Quirici N, Demarosi F, et al. Ability of polyurethane foams to support cell proliferation and the differentiation of MSCs into osteoblasts. Acta Biomater. 2009; 5:1126–1136.19. Huddleston SM, Feldman RM, Budenz DL, et al. Aqueous shunt exposure: a retrospective review of repair outcome. J Glaucoma. 2013; 22:433–438.20. El Majdoub F, Löhr M, Maarouf M, et al. Transmigration of fibrino-purulent inflammation and malignant cells into an artificial dura substitute (Neuro-Patch): report of two cases. Acta Neurochir (Wien). 2009; 151:833–835.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgical Treatment for Tube Erosion after Ahmed Valve Implantation
- Amniotic Membrane Transplantation for Tube Eerosion after Ahmed Glaucoma Valve Implant Surgery: Two Cases
- Scleral Allograft with Autologous Buccal Mucosal Transplantation for Tube Erosion Ocurred after Ahmed Glaucoma Valve Implant Surgery: Three Cases
- Ahmed Valve Implantation Using Scleral Tunneling
- Surgical Outcomes of Different Ahmed Glaucoma Valve Implantation Methods between Scleral Graft and Scleral Flap