Perinatology.
2017 Jun;28(2):59-68. 10.14734/PN.2017.28.2.59.
Comparison of Two Congenital Myotonic Dystrophy Groups According to the Number of CTG Trinucleotide Copies on Clinical Characteristics and Outcomes
- Affiliations
-
- 1Department of Pediatrics, Chonnam National University Hospital, Gwangju, Korea. essong@jnu.ac.kr
- 2Department of Pediatrics, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2386354
- DOI: http://doi.org/10.14734/PN.2017.28.2.59
Abstract
OBJECTIVE
The aims of this study were to compare clinical characteristics and outcome of neonates with congenital myotonic dystrophy (CMD) according to the number of cytosine, thymine, guanine (CTG) copies and to analyze the relating factors for survival.
METHODS
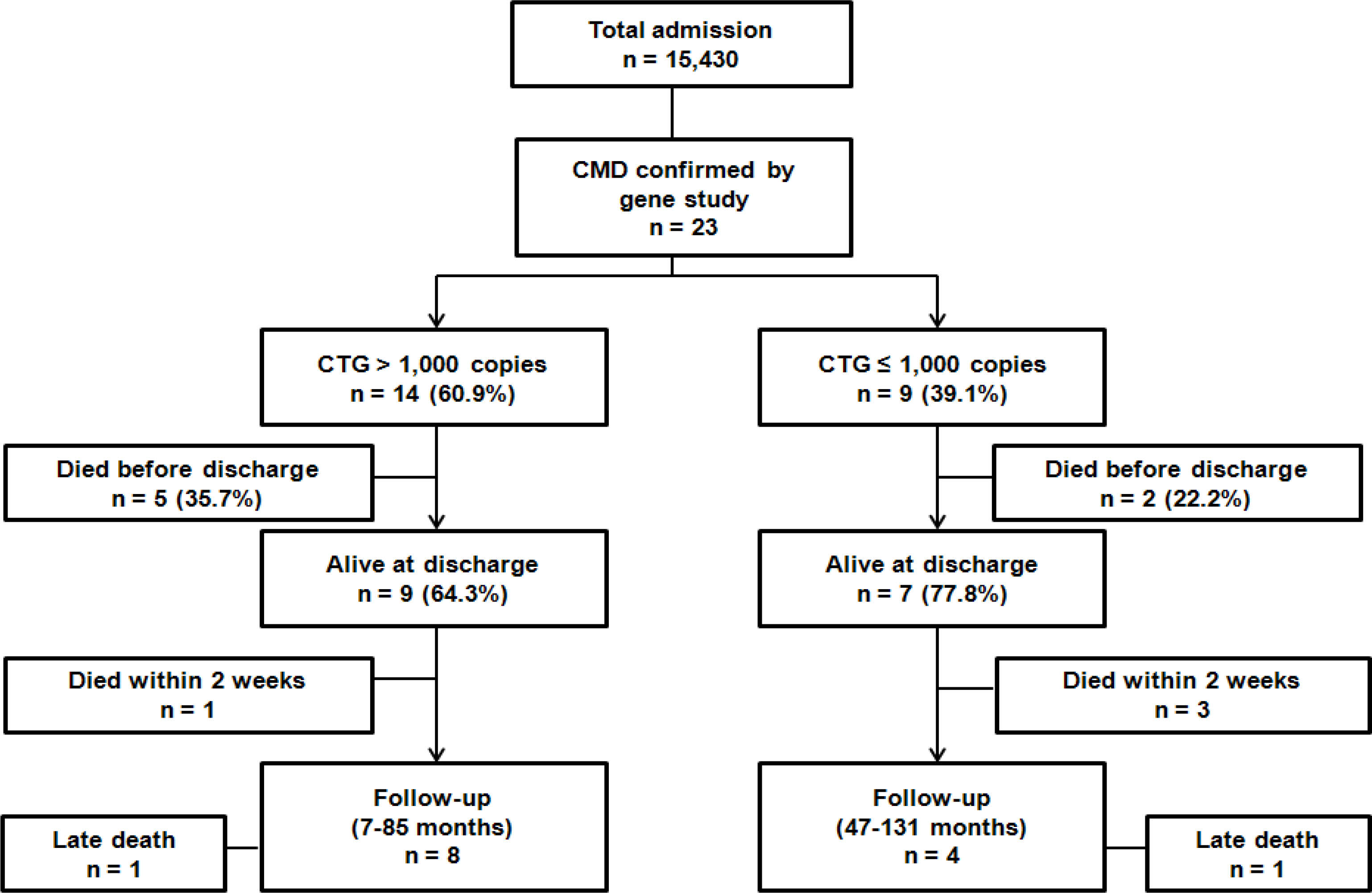
The patients were divided into two groups; less than or equal to 1,000 CTG copies (group A) and above 1,000 CTG copies (group B). This study compared the maternal CTG copies, obstetric characteristics, and patients' clinical characteristics, morbidity, hospital course, and long term outcome between group A and B, and also analyzed the relating factors for survival.
RESULTS
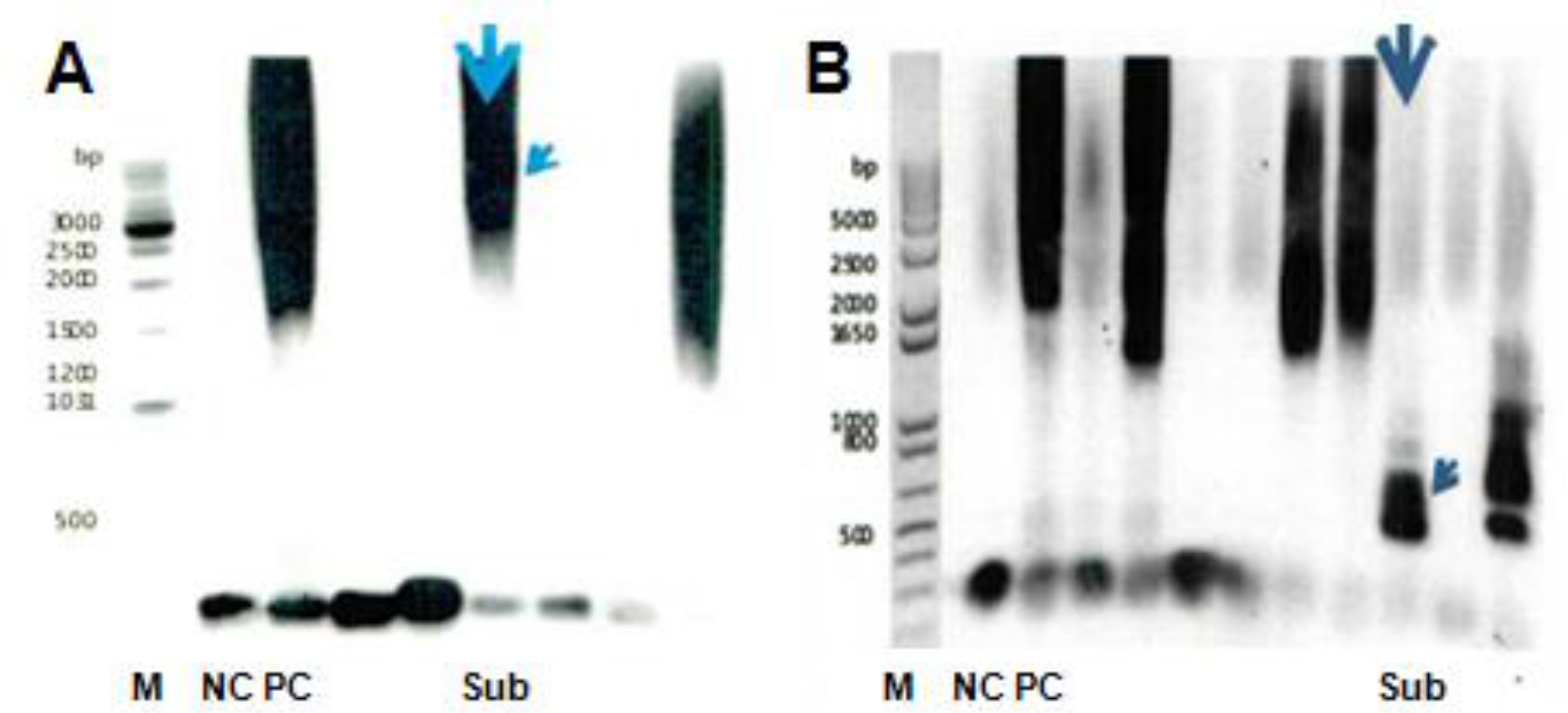
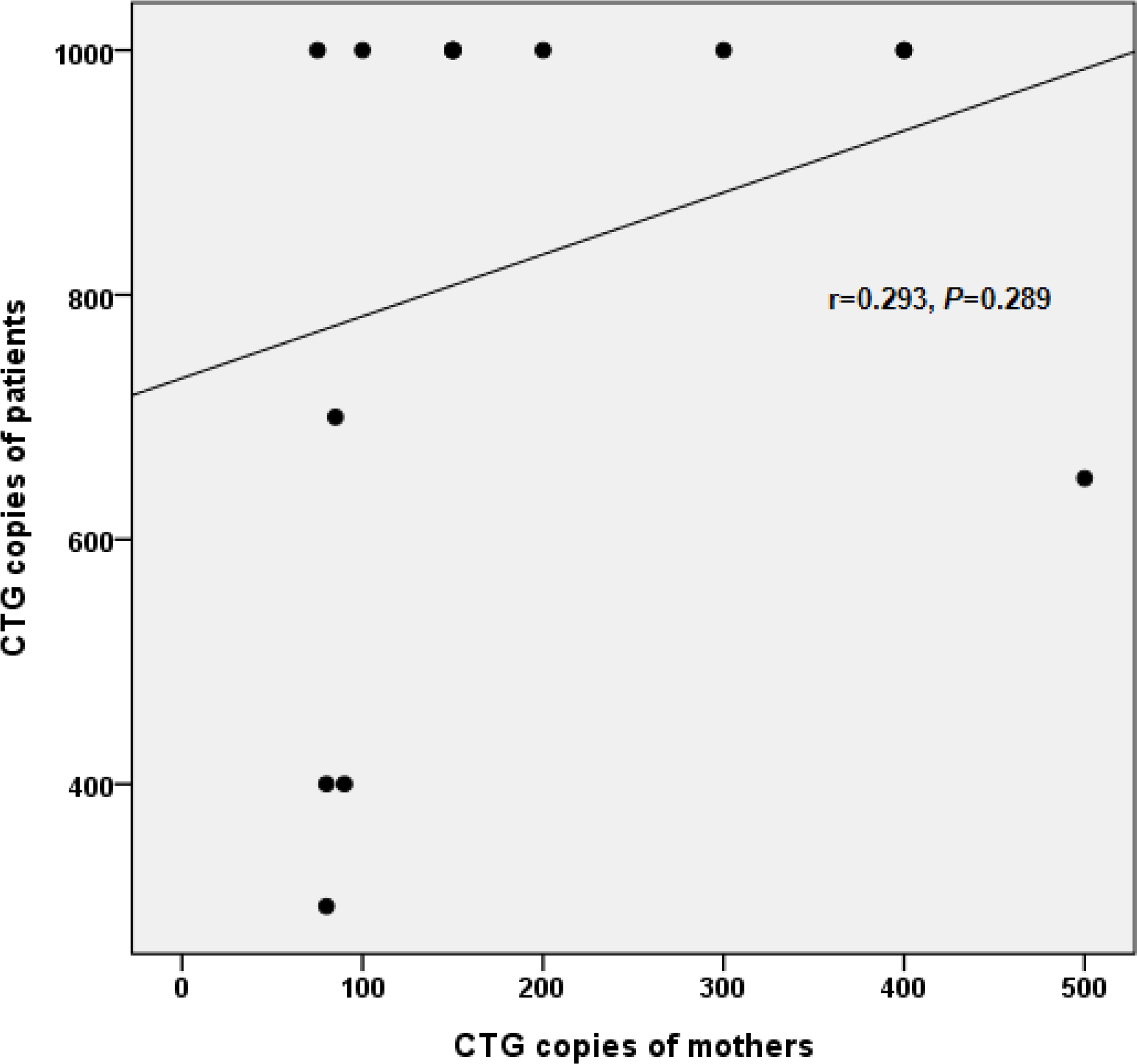
The twenty-three patients were confirmed by gene analysis in the neonatal period. Nine patients (39.1%) were included in group A and fourteen patients (60.9%) in group B. There was no correlation between the number of CTG copies of the mothers and their babies. There were no significant differences in maternal obstetric characteristics, patient's clinical findings, morbidities, hospital course and mortality between group A and B. Seven patients died before discharge and six patients among 16 who survived died after discharge. Analyzing the relating factors for survival, Apgar score at 1 and 5 minute were significantly higher in patients who survived than those who expired (P=0.0001, P=0.01, respectively). All survived patients showed developmental delay and 7 patients (58.3%) failed to thrive.
CONCLUSION
There was no correlation between the number of CTG copies of the mothers and their babies. There were no statistical differences in maternal obstetric characteristics, patient's clinical findings, morbidities, hospital course, and mortality between the two groups. Apgar score at 1 minute and 5 minute were the relating factors for survival.
MeSH Terms
Figure
Reference
-
1). Vanier TM. Dystrophia myotonica in childhood. Br Med J. 1960. 2:1284–8.
Article2). Tsilfidis C., MacKenzie AE., Mettler G., Barceló J., Korneluk RG. Correlation between CTG trinucleotide repeat length and frequency of severe congenital myotonic dystrophy. Nat Genet. 1992. 1:192–5.
Article3). Campbell C., Sherlock R., Jacob P., Blayney M. Congenital myotonic dystrophy: assisted ventilation duration and outcome. Pediatrics. 2004. 113:811–6.
Article4). de Die-Smulders CE., Smeets HJ., Loots W., Anten HB., Mirandolle JF., Geraedts JP, et al. Paternal transmission of congenital myotonic dystrophy. J Med Genet. 1997. 34:930–3.
Article5). Ranum LP., Cooper TA. RNA-mediated neuromuscular disorders. Annu Rev Neurosci. 2006. 29:259–77.
Article6). Pearson CE., Nichol Edamura K., Cleary JD. Repeat instability: mechanisms of dynamic mutations. Nat Rev Genet. 2005. 6:729–42.
Article7). Schara U., Schoser BG. Myotonic dystrophies type 1 and 2: a summary on current aspects. Semin Pediatr Neurol. 2006. 13:71–9.
Article8). Dyken PR., Harper PS. Congenital dystrophia myotonica. Neurology. 1973. 23:465–73.
Article9). Bird TD. Myotonic dystrophy type 1. [accessed on 22 Oct 2015]. Available at. http://www.ncbi.nlm.nih.gov/books/NBK1165.10). Redman JB., Fenwick RG Jr., Fu YH., Pizzuti A., Caskey CT. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993. 269:1960–5.
Article11). Kim HK., Kim JH., Lee YA., Ko TS., Kim KS., Yoo HW, et al. A case of congenital myotonic dystrophy diagnosed by molecular genetics. J Korean Child Neurol Soc. 1998. 5:356–60.12). Kim SH., Kim EY., Park SK., Choi SJ., Lim SC. A case of congenital myotonic dystrophy diagnosed by molecular genetics. J Korean Soc Neonatol. 2006. 13:194–8.13). Jung SH., Bang MS. Belated diagnosis of congenital myotonic dystrophy in a boy with cerebral palsy. Am J Phys Med Rehabil. 2007. 86:161–5.
Article14). Yum MS., Lee BH., Kim GH., Lee JJ., Choi SH., Lee JY, et al. Southern analysis after long-range PCR: clinical application in Korean patients with myotonic dystrophy 1. J Genet Med. 2013. 10:33–7.
Article15). Papile LA., Burstein J., Burstein R., Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. 92:529–34.
Article16). Walsh MC., Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986. 33:179–201.
Article17). Chang YS., Ahn SY., Park WS. Committee on program and planning and advisory committee of Korean Neonatal Network. The establishment of the Korean Neonatal Network (KNN). Neonatal Med. 2013. 20:169–78.18). Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992. 255:1253–5.
Article19). Fu YH., Pizzuti A., Fenwick RG Jr., King J., Rajnarayan S., Dunne PW, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992. 255:1256–8.
Article20). Campbell C. Congenital myotonic dystrophy. J Neurol Neurophysiol. 2012. S7:001.
Article21). Harley HG., Rundle SA., MacMillan JC., Myring J., Brook JD., Crow S, et al. Size of the unstable CTG repeat sequence in relation to phenotype and parental transmission in myotonic dystrophy. Am J Hum Genet. 1993. 52:1164–74.22). Imbert G., Kretz C., Johnson K., Mandel JL. Origin of the expansion mutation in myotonic dystrophy. Nat Genet. 1993. 4:72–6.
Article23). Groenen P., Wieringa B. Expanding complexity in myotonic dystrophy. Bioessays. 1998. 20:901–12.
Article24). Marchini C., Lonigro R., Verriello L., Pellizzari L., Bergonzi P., Damante G. Correlations between individual clinical manifestations and CTG repeat amplification in myotonic dystrophy. Clin Genet. 2000. 57:74–82.
Article25). Arsenault ME., Prévost C., Lescault A., Laberge C., Puymirat J., Mathieu J. Clinical characteristics of myotonic dystrophy type 1 patients with small CTG expansions. Neurology. 2006. 66:1248–50.
Article26). Logigian EL., Moxley RT 4th., Blood CL., Barbieri CA., Martens WB., Wiegner AW, et al. Leukocyte CTG repeat length correlates with severity of myotonia in myotonic dystrophy type 1. Neurology. 2004. 62:1081–9.
Article27). Day JW., Ranum LP. RNA pathogenesis of the myotonic dystrophies. Neuromuscul Disord. 2005. 15:5–16.
Article28). Schild RL., Plath H., Hofstaetter C., Brenner R., Mann E., Mundegar RR, et al. Polyhydramnios: an association with congenital myotonic dystrophy. J Obstet Gynaecol. 1998. 18:484–5.29). Regev R., de Vries LS., Heckmatt JZ., Dubowitz V. Cerebral ventricular dilation in congenital myotonic dystrophy. J Pediatr. 1987. 111:372–6.
Article30). Rutherford MA., Heckmatt JZ., Dubowitz V. Congenital myotonic dystrophy: respiratory function at birth determines survival. Arch Dis Child. 1989. 64:191–5.
Article31). Fujii T., Yorifuji T., Okuno T., Toyokuni S., Okada S., Mikawa H. Congenital myotonic dystrophy with progressive edema and hypoproteinemia. Brain Dev. 1991. 13:58–60.
Article32). Awater C., Zerres K., Rudnik-Schöneborn S. Pregnancy course and outcome in women with hereditary neuromuscular disorders: comparison of obstetric risks in 178 patients. Eur J Obstet Gynecol Reprod Biol. 2012. 162:153–9.
Article33). Hamza A., Herr D., Solomayer EF., Meyberg-Solomayer G. Polyhydramnios: causes, diagnosis and therapy. Geburtshilfe Frauenheilkd. 2013. 73:1241–6.
Article34). Echenne B., Rideau A., Roubertie A., Sébire G., Rivier F., Lemieux B. Myotonic dystrophy type I in childhood long-term evolution in patients surviving the neonatal period. Eur J Paediatr Neurol. 2008. 12:210–23.35). Hageman AT., Gabreëls FJ., Liem KD., Renkawek K., Boon JM. Congenital myotonic dystrophy; a report on thirteen cases and a review of the literature. J Neurol Sci. 1993. 115:95–101.
Article36). Wesström G., Bensch J., Schollin J. Congenital myotonic dystrophy. Incidence, clinical aspects and early prognosis. Acta Paediatr Scand. 1986. 75:849–54.37). Reardon W., Newcombe R., Fenton I., Sibert J., Harper PS. The natural history of congenital myotonic dystrophy: mortality and long term clinical aspects. Arch Dis Child. 1993. 68:177–81.
Article38). Roig M., Balliu PR., Navarro C., Brugera R., Losada M. Presentation, clinical course, and outcome of the congenital form of myotonic dystrophy. Pediatr Neurol. 1994. 11:208–13.
Article39). Connolly MB., Roland EH., Hill A. Clinical features for prediction of survival in neonatal muscle disease. Pediatr Neurol. 1992. 8:285–8.
Article40). Keller C., Reynolds A., Lee B., Garcia-Prats J. Congenital myotonic dystrophy requiring prolonged endotracheal and noninvasive assisted ventilation: not a uniformly fatal condition. Pediatrics. 1998. 101(4 Pt 1):704–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical characteristics of congenital myotonic dystrophy diagnosed by molecular genetic method
- The CTG Repeat Polymorphisms of Myotonic Dystrophy (DM) Gene in Korean Population
- Clinical Significance of CTG Repeat Expansion in Korean Myotonic Dystrophy Patients
- A Case of Congenital Myotonic Dystrophy Diagnosed by Molecular Genetics
- A study of trinucleotide repeat expansions in myotonic dystrophy