Korean J Crit Care Med.
2017 May;32(2):142-153. 10.4266/kjccm.2017.00094.
Implications of Plasma Renin Activity and Plasma Aldosterone Concentration in Critically Ill Patients with Septic Shock
- Affiliations
-
- 1Division of Pulmonology, Department of Internal Medicine, Institute of Chest Disease, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. PMS70@yuhs.ac
- 2Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2384039
- DOI: http://doi.org/10.4266/kjccm.2017.00094
Abstract
- BACKGROUND
The renin-angiotensin-aldosterone system is closely associated with volume status and vascular tone in septic shock. The present study aimed to assess whether plasma renin activity (PRA) and plasma aldosterone concentration (PAC) measurements compared with conventional severity indicators are associated with mortality in patients with septic shock.
METHODS
We evaluated 105 patients who were admitted for septic shock. Plasma levels of the biomarkers PRA and PAC, the PAC/PRA ratio, C-reactive protein (CRP) level, and cortisol level on days 1, 3, and 7 were serially measured. During the intensive care unit stay, relevant clinical information and laboratory results were recorded.
RESULTS
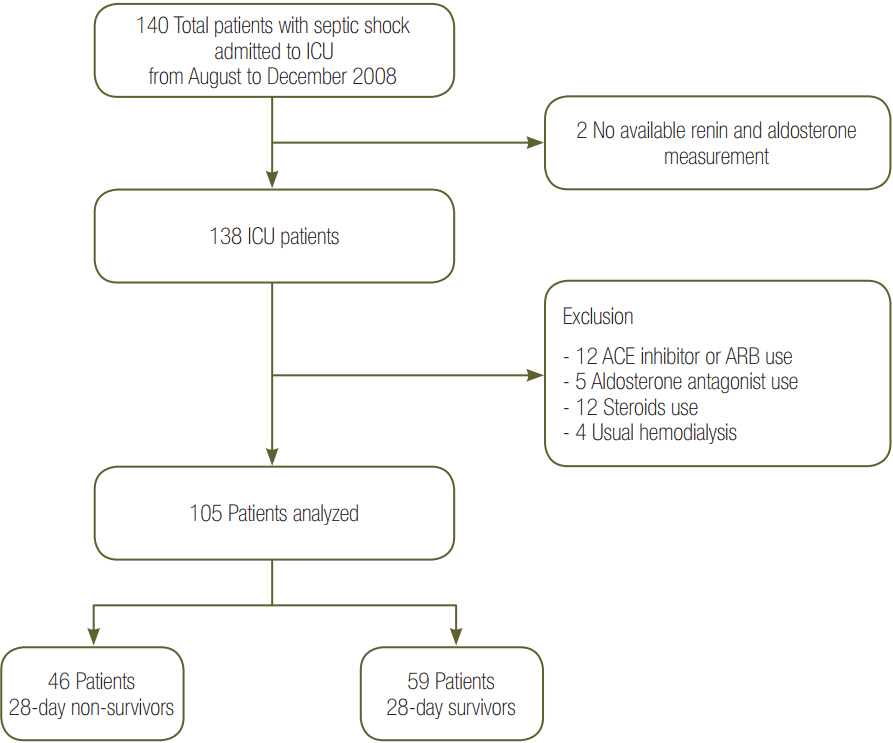
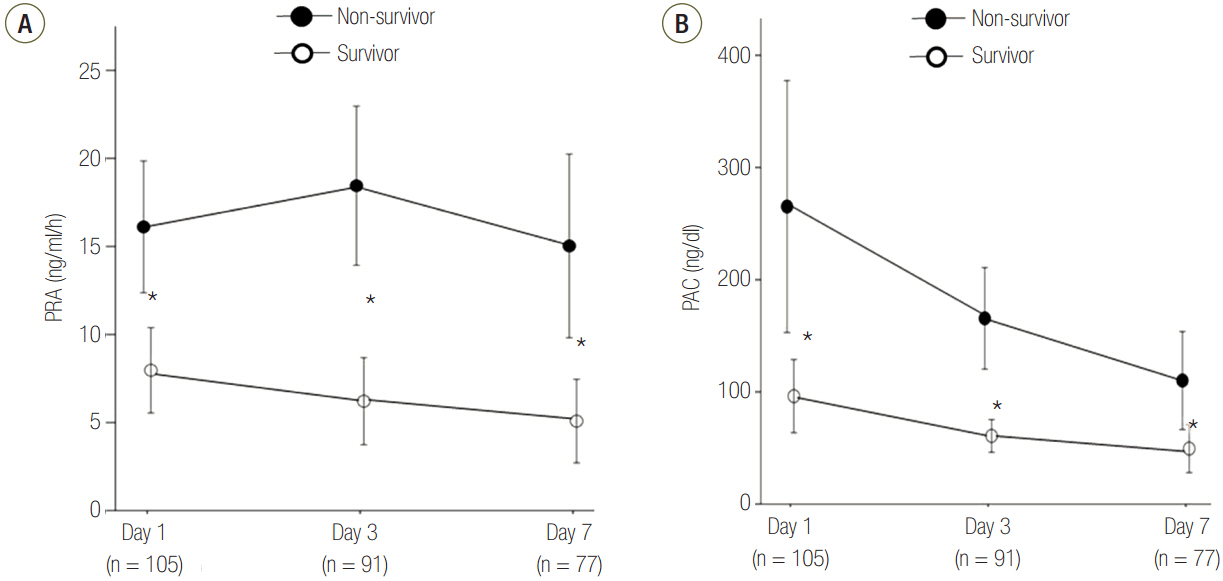
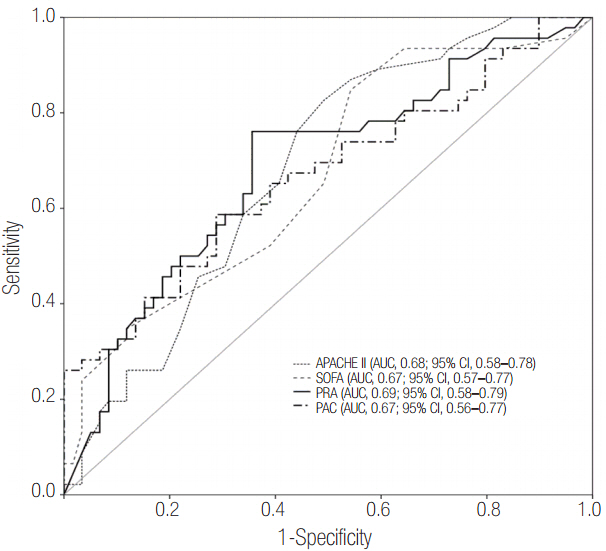
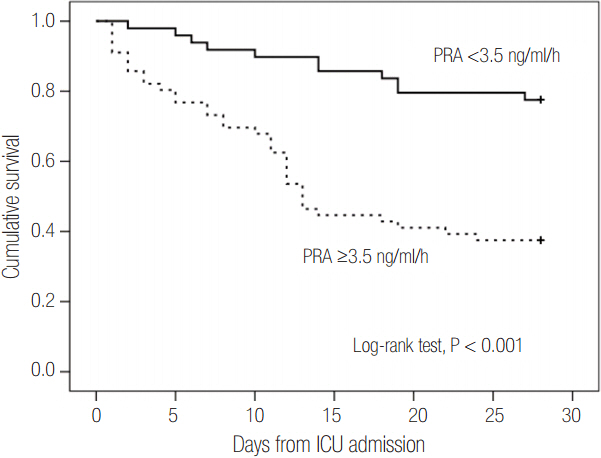
Patients were divided into two groups according to 28-day mortality: survivors (n = 59) and non-survivors (n = 46). The survivor group showed lower PRA, PAC, Acute Physiologic and Chronic Health Evaluation (APACHE) II score, and Sequential Organ Failure Assessment (SOFA) score than did the non-survivor group (all P < 0.05). The SOFA score was positively correlated with PRA (r = 0.373, P < 0.001) and PAC (r = 0.316, P = 0.001). According to receiver operating characteristic analysis, the areas under the curve of PRA and PAC to predict 28-day mortality were 0.69 (95% confidence interval [CI], 0.58 to 0.79; P = 0.001) and 0.67 (95% CI, 0.56 to 0.77; P = 0.003), respectively, similar to the APACHE II scores and SOFA scores. In particular, the group with PRA value ≥3.5 ng ml⻹ h⻹ on day 1 showed significantly greater mortality than did the group with PRA value <3.5 ng ml⻹ h⻹ (log-rank test, P < 0.001). According to multivariate analysis, SOFA score (hazard ratio, 1.11; 95% CI, 1.01 to 1.22), PRA value ≥3.5 ng ml⻹ h⻹ (hazard ratio, 3.25; 95% CI, 1.60 to 6.60), previous history of cancer (hazard ratio, 3.44; 95% CI, 1.72 to 6.90), and coronary arterial occlusive disease (hazard ratio, 2.99; 95% CI, 1.26 to 7.08) were predictors of 28-day mortality.
CONCLUSIONS
Elevated PRA is a useful biomarker to stratify the risk of critically ill patients with septic shock and is a prognostic predictor of 28-day mortality.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001; 29:1303–10.
Article2. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003; 348:1546–54.
Article3. Bone RC, Grodzin CJ, Balk RA. Sepsis: a new hypothesis for pathogenesis of the disease process. Chest. 1997; 112:235–43.
Article4. Marik PE, Zaloga GP. Adrenal insufficiency in the critically ill: a new look at an old problem. Chest. 2002; 122:1784–96.5. Annane D, Sébille V, Troché G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000; 283:1038–45.
Article6. Rothwell PM, Lawler PG. Prediction of outcome in intensive care patients using endocrine parameters. Crit Care Med. 1995; 23:78–83.
Article7. Doerschug KC, Delsing AS, Schmidt GA, Ashare A. Renin-angiotensin system activation correlates with microvascular dysfunction in a prospective cohort study of clinical sepsis. Crit Care. 2010; 14:R24.
Article8. du Cheyron D, Lesage A, Daubin C, Ramakers M, Charbonneau P. Hyperreninemic hypoaldosteronism: a possible etiological factor of septic shock-induced acute renal failure. Intensive Care Med. 2003; 29:1703–9.
Article9. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003; 29:530–8.
Article10. Cousin C, Bracquart D, Contrepas A, Nguyen G. Potential role of the (pro)renin receptor in cardiovascular and kidney diseases. J Nephrol. 2010; 23:508–13.11. Salgado DR, Rocco JR, Silva E, Vincent JL. Modulation of the renin-angiotensin-aldosterone system in sepsis: a new therapeutic approach? Expert Opin Ther Targets. 2010; 14:11–20.
Article12. Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005; 436:112–6.
Article13. Hagiwara S, Iwasaka H, Hidaka S, Hasegawa A, Koga H, Noguchi T. Antagonist of the type-1 ANG II receptor prevents against LPS-induced septic shock in rats. Intensive Care Med. 2009; 35:1471–8.
Article14. Hagiwara S, Iwasaka H, Matumoto S, Hidaka S, Noguchi T. Effects of an angiotensin-converting enzyme inhibitor on the inflammatory response in in vivo and in vitro models. Crit Care Med. 2009; 37:626–33.
Article15. Hirano Y, Takeuchi H, Suda K, Hagiwara T, Miyasho T, Kawamura Y, et al. (Pro)renin receptor blocker improves survival of rats with sepsis. J Surg Res. 2014; 186:269–77.
Article16. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010; 14:R15.
Article17. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. Early identification of intensive care unit-acquired infections with daily monitoring of C-reactive protein: a prospective observational study. Crit Care. 2006; 10:R63.18. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. C-reactive protein as a marker of ventilator-associated pneumonia resolution: a pilot study. Eur Respir J. 2005; 25:804–12.19. Póvoa P, Coelho L, Almeida E, Fernandes A, Mealha R, Moreira P, et al. Pilot study evaluating C-reactive protein levels in the assessment of response to treatment of severe bloodstream infection. Clin Infect Dis. 2005; 40:1855–7.20. Póvoa P, Teixeira-Pinto AM, Carneiro AH; Portuguese Community-Acquired Sepsis Study Group SACiUCI. C-reactive protein, an early marker of community-acquired sepsis resolution: a multicenter prospective observational study. Crit Care. 2011; 15:R169.
Article21. du Cheyron D, Bouchet B, Cauquelin B, Guillotin D, Ramakers M, Daubin C, et al. Hyperreninemic hypoaldosteronism syndrome, plasma concentrations of interleukin-6 and outcome in critically ill patients with liver cirrhosis. Intensive Care Med. 2008; 34:116–24.
Article22. Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003; 144:2179–83.
Article23. Liu HQ, Wei XB, Sun R, Cai YW, Lou HY, Wang JW, et al. Angiotensin II stimulates intercellular adhesion molecule-1 via an AT1 receptor/nuclear factor-kappaB pathway in brain microvascular endothelial cells. Life Sci. 2006; 78:1293–8.24. Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, et al. Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl. 2002; (82):S12-22.
Article25. Chai SY, Fernando R, Peck G, Ye SY, Mendelsohn FA, Jenkins TA, et al. The angiotensin IV/AT4 receptor. Cell Mol Life Sci. 2004; 61:2728–37.26. Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin Sci (Lond). 2007; 112:417–28.
Article27. Newton CR, Curran B, Victorino GP. Angiotensin II type 1 receptor activation increases microvascular permeability via a calcium dependent process. J Surg Res. 2005; 123:33–9.28. Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, et al. Prognostic value of plasma renin activity in heart failure. Am J Cardiol. 2011; 108:246–51.
Article29. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013; 39:165–228.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on the Change of Plasma Renin Activity(PRA) and Aldosterone Concentration(PAC) before and after Heart Operation in Children with Congenital Heart Disease
- A study of plasma renin activity and plasma aldosterone in chronic liver diseases
- Anesthetic Experience with Primary Aldosteronism: Report of a case
- Two Cases with Pseudohypoaldosteronism
- Hemodynamic changes after single large volume paracentesis(SLVP) in cirrhosis with tense ascites: Focusing on the effect of albumin as a plasma expander