Ann Rehabil Med.
2017 Feb;41(1):113-120. 10.5535/arm.2017.41.1.113.
Effect of Intravenous Infusion of G-CSF-Mobilized Peripheral Blood Mononuclear Cells on Upper Extremity Function in Cerebral Palsy Children
- Affiliations
-
- 1Department of Rehabilitation Medicine, Hanyang University College of Medicine, Seoul, Korea. kimmjreh@hanyang.ac.kr
- 2Department of Pediatrics, Hanyang University Medical Center, Seoul, Korea.
- 3Department of Translational Medicine, Graduate School of Biomedical Engineering, Hanyang University, Seoul, Korea.
- 4Blood & Marrow Transplantation Center, Hanyang University Medical Center, Seoul, Korea.
- KMID: 2383685
- DOI: http://doi.org/10.5535/arm.2017.41.1.113
Abstract
OBJECTIVE
To investigate the effect of intravenous infusion of peripheral blood mononuclear cells (mPBMC) mobilized by granulocyte-colony stimulating factor (G-CSF) on upper extremity function in children with cerebral palsy (CP).
METHODS
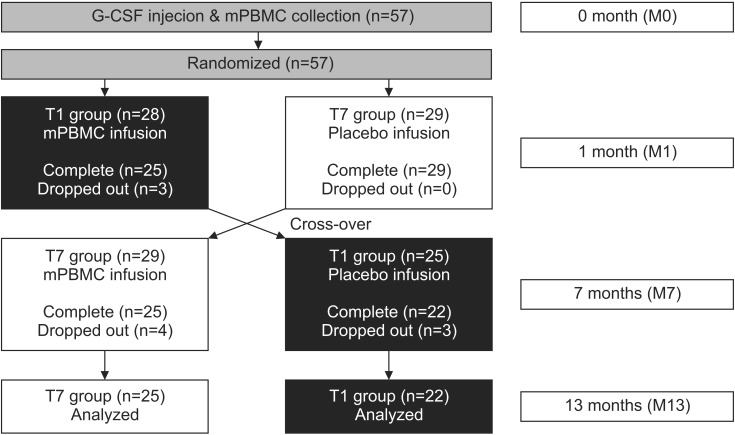
Fifty-seven children with CP were enrolled. Ten patients were excluded due to follow-up loss. In total, 47 patients (30 males and 17 females) were analyzed. All patients' parents provided signed consent before the start of the study. After administration of G-CSF for 5 days, mPBMC was collected and cryopreserved. Patients were randomized into two groups 1 month later. Twenty-two patients were administered mPBMC and 25 patients received normal saline as placebo. Six months later, the two groups were switched, and administered mPBMC and placebo, respectively. Quality of Upper Extremity Skills Test (QUEST) and the Manual Ability Classification System (MACS) were used to evaluate upper motor function.
RESULTS
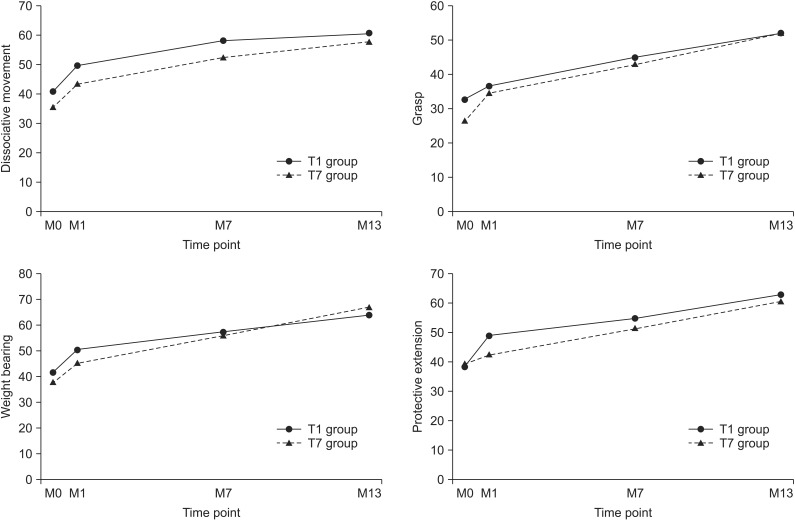
All subdomain and total scores of QUEST were significantly improved after mPBMC and placebo infusion, without significant differences between mPBMC and placebo groups. A month after G-CSF, all subdomain and total scores of QUEST were improved. The level of MACS remained unchanged in both mPBMC and placebo groups.
CONCLUSION
In this study, intravenously infused mPBMC showed no significant effect on upper extremity function in children with CP, as compared to placebo. The effect of mPBMC was likely masked by the effect of G-CSF, which was used in both groups and/or G-CSF itself might have other neurotrophic potentials in children with CP.
Keyword
MeSH Terms
Figure
Reference
-
1. Colver A, Fairhurst C, Pharoah PO. Cerebral palsy. Lancet. 2014; 383:1240–1249. PMID: 24268104.
Article2. Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004; 363:1619–1631. PMID: 15145637.
Article3. McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol. 2013; 55:499–508. PMID: 23181910.
Article4. Mancias-Guerra C, Marroquin-Escamilla AR, Gonzalez-Llano O, Villarreal-Martinez L, Jaime-Perez JC, Garcia-Rodriguez F, et al. Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: an open-label phase I trial. Cytotherapy. 2014; 16:810–820. PMID: 24642016.
Article5. Wang X, Cheng H, Hua R, Yang J, Dai G, Zhang Z, et al. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study. Cytotherapy. 2013; 15:1549–1562. PMID: 24100132.
Article6. Purandare C, Shitole DG, Belle V, Kedari A, Bora N, Joshi M. Therapeutic potential of autologous stem cell transplantation for cerebral palsy. Case Rep Transplant. 2012; 2012:825289. PMID: 23259143.
Article7. Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, Wang JP, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011; 32:641–651. PMID: 21678074.
Article8. Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K. Evidence of peripheral blood-derived, plastic-adherent CD34(−/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells. 2000; 18:252–260. PMID: 10924091.9. Law M, Cadman D, Rosenbaum P, Walter S, Russell D, DeMatteo C. Neurodevelopmental therapy and upper-extremity inhibitive casting for children with cerebral palsy. Dev Med Child Neurol. 1991; 33:379–387. PMID: 2065824.
Article10. Thorley M, Lannin N, Cusick A, Novak I, Boyd R. Reliability of the quality of upper extremity skills test for children with cerebral palsy aged 2 to 12 years. Phys Occup Ther Pediatr. 2012; 32:4–21. PMID: 21838618.
Article11. Jeevanantham D, Dyszuk E, Bartlett D. The manual ability classification system: a scoping review. Pediatr Phys Ther. 2015; 27:236–241. PMID: 26020598.12. Alvarez P, Carrillo E, Velez C, Hita-Contreras F, Martinez-Amat A, Rodriguez-Serrano F, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. Biomed Res Int. 2013; 2013:312656. PMID: 23844360.
Article13. Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999; 96:10711–10716. PMID: 10485891.
Article14. Faulkner SD, Ruff CA, Fehlings MG. The potential for stem cells in cerebral palsy: piecing together the puzzle. Semin Pediatr Neurol. 2013; 20:146–153. PMID: 23948689.15. Brenneman M, Sharma S, Harting M, Strong R, Cox CS Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010; 30:140–149. PMID: 19773802.
Article16. Carroll JE, Mays RW. Update on stem cell therapy for cerebral palsy. Expert Opin Biol Ther. 2011; 11:463–471. PMID: 21299445.
Article17. Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg. 2009; 138:646–653. PMID: 19698851.
Article18. Sharma A, Sane H, Gokulchandran N, Kulkarni P, Gandhi S, Sundaram J, et al. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: a new frontier. Stem Cells Int. 2015; 2015:905874. PMID: 25788947.
Article19. Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005; 66:543–551. PMID: 15914119.
Article20. Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007; 24:508–520. PMID: 17402856.
Article21. Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005; 4:1753–1757. PMID: 16258290.22. Moon JH, Kim MJ, Song SY, Lee YJ, Choi YY, Kim SH, et al. Safety and efficacy of G-CSF mobilization and collection of autologous peripheral blood stem cells in children with cerebral palsy. Transfus Apher Sci. 2013; 49:516–521. PMID: 24035522.
Article23. Koh H, Hwang K, Lim HY, Kim YJ, Lee YH. Mononuclear cells from the cord blood and granulocytecolony stimulating factor-mobilized peripheral blood: is there a potential for treatment of cerebral palsy? Neural Regen Res. 2015; 10:2018–2024. PMID: 26889193.
Article24. England TJ, Abaei M, Auer DP, Lowe J, Jones DR, Sare G, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012; 43:405–411. PMID: 22198983.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serial Changes of Cytokines in Children with Cerebral Palsy Who Received Intravenous Granulocyte-colony Stimulating Factor Followed by Autologous Mobilized Peripheral Blood Mononuclear Cells
- The Effects of Single Event Multi-level Chemoneurolysis on Upper Extremity Function in Children with Cerebral Palsy
- Relation among the Gross Motor Function, Manual Performance and Upper Limb Functional Measures in Children with Spastic Cerebral Palsy
- Effects of Hematopoietic Growth Factors on the Bone Marrow and Mobilized Peripheral Progenitors from the Same Person
- Peripheral Blood Mononuclear Cells and Growth Factor Therapy for Cerebral Palsy