Ann Dermatol.
2016 Oct;28(5):586-592. 10.5021/ad.2016.28.5.586.
Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model
- Affiliations
-
- 1Department of Dermatology, St. Paul's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. johnkang@catholic.ac.kr
- 2Department of Dermatology, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2382883
- DOI: http://doi.org/10.5021/ad.2016.28.5.586
Abstract
- BACKGROUND
Microneedle is a method that creates transdermal microchannels across the stratum corneum barrier layer of skin. No previous study showed a therapeutic effect of microneedle itself on hair growth by wounding.
OBJECTIVE
The aim of this study is to investigate the effect of repeated microwound formed by microneedle on hair growth and hair growth-related genes in a murine model.
METHODS
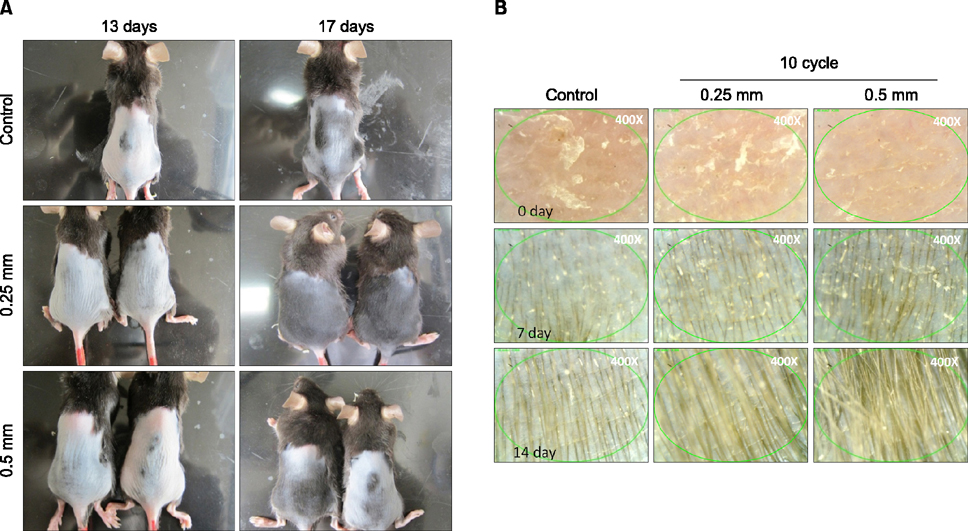
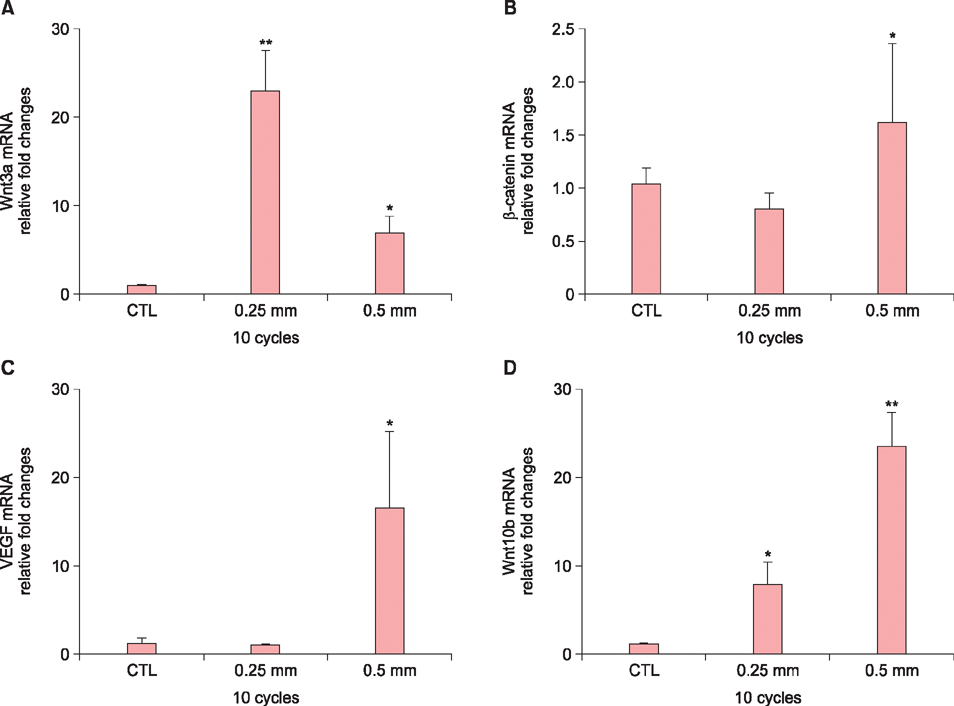
A disk microneedle roller was applied to each group of mice five times a week for three weeks. First, to identify the optimal length and cycle, microneedles of lengths of 0.15 mm, 0.25 mm, 0.5 mm, and 1 mm and cycles of 3, 6, 10, and 13 cycles were applied. Second, the effect of hair growth and hair-growth-related genes such as Wnt3a, β-catenin, vascular endothelial growth factor (VEGF), and Wnt10b was observed using optimized microneedle. Outcomes were observed using visual inspection, real-time polymerase chain reaction, and immunohistochemistry.
RESULTS
We found that the optimal length and cycle of microneedle treatment on hair growth was 0.25 mm/10 cycles and 0.5 mm/10 cycles. Repeated microneedle stimulation promoted hair growth, and it also induced the enhanced expression of Wnt3a, β-catenin, VEGF, and Wnt10b.
CONCLUSION
Our study provides evidence that microneedle stimulation can induce hair growth via activation of the Wnt/β-catenin pathway and VEGF. Combined with the drug delivery effect, we believe that microneedle stimulation could lead to new approaches for alopecia.
Keyword
MeSH Terms
Figure
Reference
-
1. Park JH, Choi SO, Seo S, Choy YB, Prausnitz MR. A microneedle roller for transdermal drug delivery. Eur J Pharm Biopharm. 2010; 76:282–289.
Article2. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004; 56:581–587.
Article3. Park KY, Jang WS, Lim YY, Ahn JH, Lee SJ, Kim CW, et al. Safety evaluation of stamp type digital microneedle devices in hairless mice. Ann Dermatol. 2013; 25:46–53.
Article4. Kim HM, Lim YY, An JH, Kim MN, Kim BJ. Transdermal drug delivery using disk microneedle rollers in a hairless rat model. Int J Dermatol. 2012; 51:859–863.
Article5. Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002; 118:216–225.
Article6. Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the "hair growth-wound healing connection": anagen phase promotes wound re-epithelialization. J Invest Dermatol. 2011; 131:518–528.
Article7. Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008; 128:1311–1318.
Article8. Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007; 447:316–320.
Article9. Alonso L, Fuchs E. The hair cycle. J Cell Sci. 2006; 119:391–393.
Article10. Yoo KH, Lee JW, Li K, Kim BJ, Kim MN. Photodynamic therapy with methyl 5-aminolevulinate acid might be ineffective in recalcitrant alopecia totalis regardless of using a microneedle roller to increase skin penetration. Dermatol Surg. 2010; 36:618–622.
Article11. Lee YB, Eun YS, Lee JH, Cheon MS, Park YG, Cho BK, et al. Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol. 2013; 40:81–83.
Article12. Leirós GJ, Attorresi AI, Balañá ME. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br J Dermatol. 2012; 166:1035–1042.
Article13. Myung PS, Takeo M, Ito M, Atit RP. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J Invest Dermatol. 2013; 133:31–41.
Article14. Ouji Y, Nakamura-Uchiyama F, Yoshikawa M. Canonical Wnts, specifically Wnt-10b, show ability to maintain dermal papilla cells. Biochem Biophys Res Commun. 2013; 438:493–499.
Article15. Guo H, Yang K, Deng F, Ye J, Xing Y, Li Y, et al. Wnt3a promotes melanin synthesis of mouse hair follicle melanocytes. Biochem Biophys Res Commun. 2012; 420:799–804.
Article16. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003; 83:835–870.
Article17. Yano K, Brown LF, Detmar M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. 2001; 107:409–417.
Article18. Hyun MY, Suk JM, Yoo KH, Kim BJ, Kim MN, Hong CK. Unwanted hair growth induced by topical epidermal growth factor during wound healing: true or myth? Int Wound J. 2014; DOI: 10.1111/iwj.12354. [Epub ahead of print].
Article19. Mohammed YH, Yamada M, Lin LL, Grice JE, Roberts MS, Raphael AP, et al. Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS One. 2014; 9:e101956.
Article20. Fernandes D, Signorini M. Combating photoaging with percutaneous collagen induction. Clin Dermatol. 2008; 26:192–199.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effects of Growth Factor Cocktail Treatment in Patients with Androgenetic Alopecia According to the Depth of Microneedle
- Therapeutic Effects of Growth Factor Cocktail Including Fibroblast Growth Factor 9 in Patients with Pattern Hair Loss
- Effects of TNF-alpha and Minoxidil on Human Hair Growth in Vitro
- Udenafil Induces the Hair Growth Effect of Adipose-Derived Stem Cells
- Alopecia