Intest Res.
2017 Jul;15(3):345-351. 10.5217/ir.2017.15.3.345.
Japanese physicians' attitudes towards enteral nutrition treatment for pediatric patients with Crohn's disease: a questionnaire survey
- Affiliations
-
- 1Department of Pediatrics, Gunma University Graduate School of Medicine, Maebashi, Japan. ishiget@gunma-u.ac.jp
- 2PAL Children's Clinic, Isesaki, Japan.
- 3Department of Pediatrics, Osaka General Medical Center, Osaka, Japan.
- 4Department of Pediatrics, Osaka Medical College, Takatsuki, Japan.
- KMID: 2382378
- DOI: http://doi.org/10.5217/ir.2017.15.3.345
Abstract
- BACKGROUND/AIMS
Enteral nutrition (EN) is recommended for the treatment of pediatric Crohn's disease (CD) in Japan. However, the indications and treatment protocols for EN vary among hospitals. In the present study, we aimed to determine how EN was administered to pediatric patients and whether physicians followed treatment guidelines in their practices.
METHODS
Two types of questionnaires were administered to 32 physicians who were involved in the treatment of pediatric CD. The consensus questionnaire evaluated the physicians' attitudes towards EN, whereas the efficacy questionnaire collected data on patients with CD, aged <17 years, who had undergone induction therapy between 2006 and 2011.
RESULTS
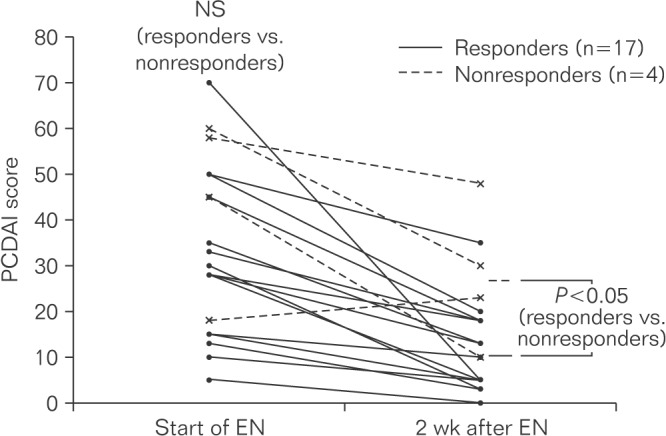
A total of 23 physicians responded to the questionnaires. The results of the consensus questionnaire indicated that 82% and 59% of study participants recommended EN treatment for all newly diagnosed pediatric patients with CD and all relapsed patients, respectively. Exclusive EN (EEN) and elemental formula were recommended by 84% and 85% of physicians, respectively. The efficacy questionnaire revealed that 57 of the 58 patients received EN. Elemental formula was used in 39 of 40 patients who were treated with EEN. Of these 40 patients, 27 were treated with EEN alone; of these, 22 (81%) achieved remission without any other treatment. The mean duration of EEN was 15.9 days.
CONCLUSIONS
EN is widely recommended by physicians treating pediatric CD in Japan. In contrast to Western countries, clinicians used elemental formula more often and with a shorter EEN treatment duration.
MeSH Terms
Figure
Reference
-
1. Ishige T, Tomomasa T, Takebayashi T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol. 2010; 45:911–917. PMID: 20232217.
Article2. Heuschkel R, Salvestrini C, Beattie RM, Hildebrand H, Walters T, Griffiths A. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008; 14:839–849. PMID: 18266237.
Article3. Peters H. Parenteral-peroral combined treatement of Crohn's disease and ulcerative colitis. Infusionsther Klin Ernahr. 1976; 3:222–226. PMID: 821860.4. Day AS, Whitten KE, Lemberg DA, et al. Exclusive enteral feeding as primary therapy for Crohn's disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol. 2006; 21:1609–1614. PMID: 16928225.
Article5. Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn's disease: pathogenesis and interventions. Inflamm Bowel Dis. 2007; 13:620–628. PMID: 17262806.6. Day AS, Stephenson T, Stewart M, Otley AR. Exclusive enteral nutrition for children with Crohn's disease: use in Australia and attitudes of Australian paediatric gastroenterologists. J Paediatr Child Health. 2009; 45:337–341. PMID: 19490411.
Article7. Stewart M, Day AS, Otley A. Physician attitudes and practices of enteral nutrition as primary treatment of paediatric Crohn disease in North America. J Pediatr Gastroenterol Nutr. 2011; 52:38–42. PMID: 20975582.
Article8. Levine A, Milo T, Buller H, Markowitz J. Consensus and controversy in the management of pediatric Crohn disease: an international survey. J Pediatr Gastroenterol Nutr. 2003; 36:464–469. PMID: 12658036.
Article9. Whitten KE, Rogers P, Ooi CY, Day AS. International survey of enteral nutrition protocols used in children with Crohn's disease. J Dig Dis. 2012; 13:107–112. PMID: 22257479.
Article10. Day AS, Whitten KE, Sidler M, Lemberg DA. Systematic review: nutritional therapy in paediatric Crohn's disease. Aliment Pharmacol Ther. 2008; 27:293–307. PMID: 18045244.
Article11. Yamamoto T, Nakahigashi M, Saniabadi AR. Review article. Diet and inflammatory bowel disease: epidemiology and treatment. Aliment Pharmacol Ther. 2009; 30:99–112. PMID: 19438426.
Article12. Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum. 2000; 43(10 Suppl):S85–S93. PMID: 11052483.13. Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis. 2014; 8:1179–1207. PMID: 24909831.14. Working Group of the Japanese Society for Pediatric Gastroenterology, Hepatology and Nutrition. Konno M, Kobayashi A, et al. Guidelines for the treatment of Crohn's disease in children. Pediatr Int. 2006; 48:349–352. PMID: 16732811.
Article15. Ueno F, Matsui T, Matsumoto T, et al. Evidence-based clinical practice guidelines for Crohn's disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013; 48:31–72. PMID: 23090001.
Article16. Giaffer MH, North G, Holdsworth CD. Controlled trial of polymeric versus elemental diet in treatment of active Crohn's disease. Lancet. 1990; 335:816–819. PMID: 1969560.
Article17. Matsui T, Sakurai T, Yao T. Nutritional therapy for Crohn's disease in Japan. J Gastroenterol. 2005; 40(Suppl 16):25–31. PMID: 15902960.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Exclusive Enteral Nutrition for the Treatment of Pediatric Crohn’s Disease: The Patient Perspective
- Effect of Short-Term Partial Enteral Nutrition on the Treatment of Younger Patients with Severe Crohn's Disease
- How do physicians and nurses differ in their perceived barriers to effective enteral nutrition in the intensive care unit?
- A Nationwide Survey on Enteral Nutrition
- Enteral Nutrition in Pediatric Patients