J Pathol Transl Med.
2015 May;49(3):243-248. 10.4132/jptm.2015.03.30.
Smad1 Expression in Follicular Lymphoma
- Affiliations
-
- 1Department of Pathology, Dankook University College of Medicine, Cheonan, Korea. cyjy555@ hanmail.net
- KMID: 2381383
- DOI: http://doi.org/10.4132/jptm.2015.03.30
Abstract
- BACKGROUND
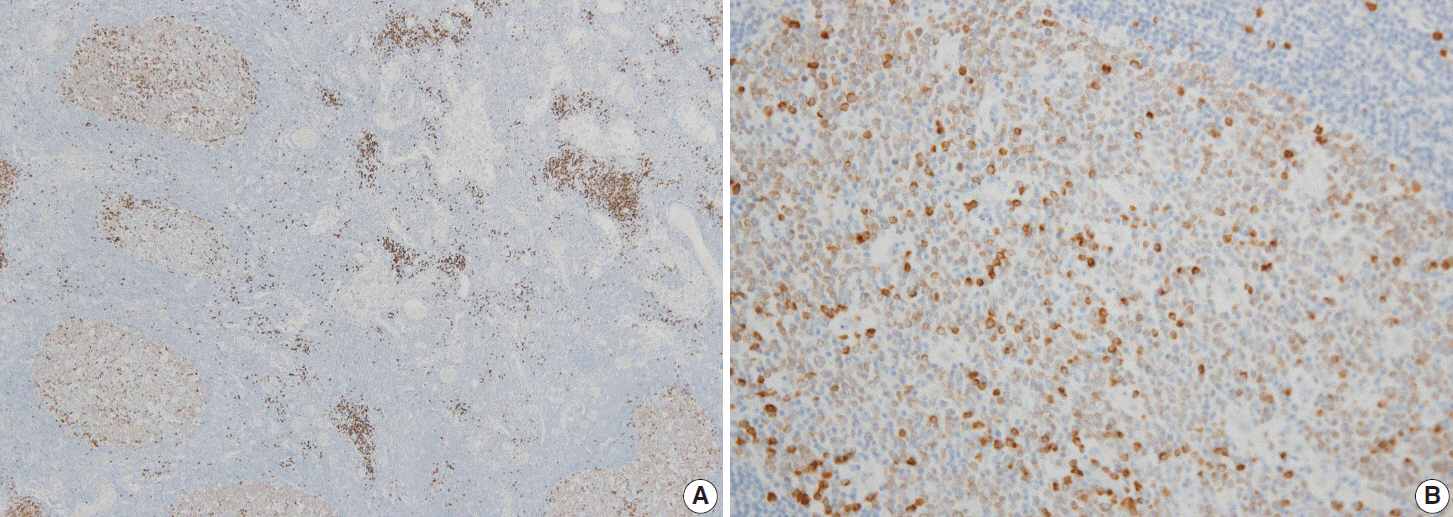
Follicular lymphomas present with various immunohistologic patterns. The immunohistochemical markers used in the diagnosis of follicular lymphoma show variable degrees of sensitivity and specificity, and thus, additional germinal center markers are required. Smad1 has been reported to be overexpressed in follicular lymphoma, but little is known regarding the expression patterns of Smad proteins in human lymphoid tissue.
METHODS
In the present study, we performed immunohistochemistry for traditional germinal center markers and for Smad1 in human reactive lymphoid and follicular lymphoma tissues to investigate Smad1's usefulness in the diagnosis of follicular lymphoma.
RESULTS
In the reactive germinal centers, most cells were positive for Smad1. Among the 27 follicular lymphoma cases, 17 of 21 (80%) were Smad1 positive, 17 of 27 (63%) were positive for CD10, and 23 of 27 (85%) were positive for Bcl6. Notably, three cases expressed CD10 only, and one only expressed Bcl6. All these cases were grade 3 tumors and showed follicular and diffuse growth patterns.
CONCLUSIONS
These results indicate that Smad1 is a candidate as a germinal center marker. Furthermore, they suggest that the Smad signaling pathway might be involved in follicular lymphoma.
Keyword
MeSH Terms
Figure
Reference
-
1. Younes SF, Beck AH, Lossos IS, Levy R, Warnke RA, Natkunam Y. Immunoarchitectural patterns in follicular lymphoma: efficacy of HGAL and LMO2 in the detection of the interfollicular and diffuse components. Am J Surg Pathol. 2010; 34:1266–76.
Article2. Kwon MS, Go JH, Choi JS, et al. Critical evaluation of Bcl-6 protein expression in diffuse large B-cell lymphoma of the stomach and small intestine. Am J Surg Pathol. 2003; 27:790–8.
Article3. King BE, Chen C, Locker J, et al. Immunophenotypic and genotypic markers of follicular center cell neoplasia in diffuse large B-cell lymphomas. Mod Pathol. 2000; 13:1219–31.
Article4. Borovecki A, Korać P, Nola M, Ivanković D, Jaksić B, Dominis M. Prognostic significance of B-cell differentiation genes encoding proteins in diffuse large B-cell lymphoma and follicular lymphoma grade 3. Croat Med J. 2008; 49:625–35.
Article5. Ree HJ, Yang WI, Kim CW, et al. Coexpression of Bcl-6 and CD10 in diffuse large B-cell lymphomas: significance of Bcl-6 expression patterns in identifying germinal center B-cell lymphoma. Hum Pathol. 2001; 32:954–62.
Article6. Husson H, Carideo EG, Neuberg D, et al. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002; 99:282–9.
Article7. Harris NL, Swerdlow SH, Jaffe ES, et al. Follicular lymphoma. Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press;2008. p. 220–6.8. Straka C, Mielke B, Eichelmann A, Trede I, Ho AD, Möller P. Bcl-2 gene rearrangements in primary B-cell lymphoma of the gastrointestinal tract reveal follicular lymphoma as a subtype. Leukemia. 1993; 7:268–73.9. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000; 403:503–11.
Article10. Eshoa C, Perkins S, Kampalath B, Shidham V, Juckett M, Chang CC. Decreased CD10 expression in grade III and in interfollicular infiltrates of follicular lymphomas. Am J Clin Pathol. 2001; 115:862–7.
Article11. Goteri G, Lucarini G, Zizzi A, et al. Comparison of germinal center markers CD10, BCL6 and human germinal center-associated lymphoma (HGAL) in follicular lymphomas. Diagn Pathol. 2011; 6:97.
Article12. Yue J, Frey RS, Mulder KM. Cross-talk between the Smad1 and Ras/MEK signaling pathways for TGFbeta. Oncogene. 1999; 18:2033–7.13. Kretzschmar M, Liu F, Hata A, Doody J, Massagué J. The TGF-beta family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 1997; 11:984–95.
Article14. Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1-6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-betas. Dev Dyn. 2001; 220:141–54.15. Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood. 1999; 94:2854–61.16. Munoz O, Fend F, de Beaumont R, Husson H, Astier A, Freedman AS. TGFbeta-mediated activation of Smad1 in B-cell non-Hodgkin’s lymphoma and effect on cell proliferation. Leukemia. 2004; 18:2015–25.17. Go JH. Expression pattern of Smad proteins in diffuse large B-cell lymphomas. Korean J Pathol. 2004; 38:301–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of bcl-2/IgH Gene Rearrangement and Expression of c-myc and p53 Oncoprotein in B-cell Lymphoma
- Dermatomyositis Associated with Follicular Lymphoma
- Composite follicular lymphoma and classic Hodgkin lymphoma
- A Rare Case of Primary Duodenal Follicular Lymphoma
- Gastric Follicular Lymphomas Presenting as Subepithelial Tumors: Two Cases