Obstet Gynecol Sci.
2017 May;60(3):314-317. 10.5468/ogs.2017.60.3.314.
Migration of a contraceptive subdermal device into the lung
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. sihyuncho@yuhs.ac
- 2Department of Chest Thoracic Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2378595
- DOI: http://doi.org/10.5468/ogs.2017.60.3.314
Abstract
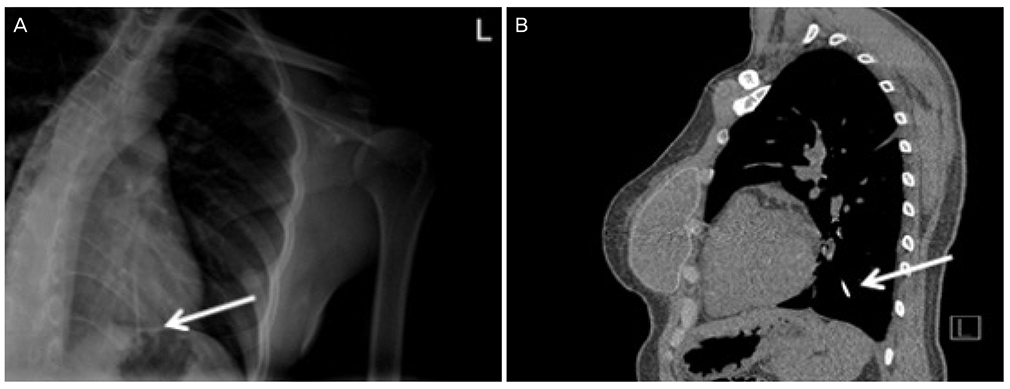
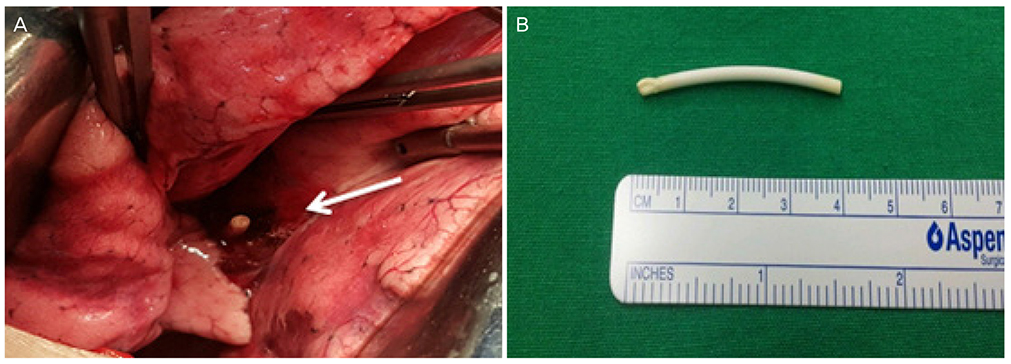
- A single-rod subdermal contraceptive implant is usually located around the insertion site, has been usually known to migrate within less than 2 cm of the insertion site and the true migration over 2 cm has been rarely reported. We report a case of migrated radiopaque subdermal contraceptive implant into lung in a 37-year-old woman. On conducted chest computed tomography, subdermal contraceptive implant was in subsegmental branch in left posterior basal segment of lung. Removal of subdermal contraceptive implant in left posterior basal segment of lung by mini-thoracotomy was performed by a chest surgeon. Complications with insertion and removal of subdermal contraceptive implant are rare in the hands of medical professionals familiar with the techniques and these procedures should only be undertaken by those with relevant training. The migration over 2 cm should not occur if the correct subdermal insertion procedure is followed and carried out by a properly trained individual.
Figure
Cited by 1 articles
-
Experiences of localization and removal of non-palpable subdermal contraceptive implants with ultrasound
SooHyun Kim, Young Sik Choi, Jeong Sook Kim, Sungjun Kim, SiHyun Cho
Obstet Gynecol Sci. 2019;62(3):166-172. doi: 10.5468/ogs.2019.62.3.166.
Reference
-
1. Balogun OR, Olaomo N, Adeniran AS, Fawole AA. Implanon sub-dermal implant: an emerging method of contraception in Ilorin, Nigeria. J Med Biomed Sci. 2014; 3:1–5.2. Croxatto HB, Urbancsek J, Massai R, Coelingh Bennink H, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999; 14:976–981.3. Collins DC. Sex hormone receptor binding, progestin selectivity, and the new oral contraceptives. Am J Obstet Gynecol. 1994; 170:1508–1513.4. Brache V, Faundes A, Alvarez F. Risk-benefit effects of implantable contraceptives in women. Expert Opin Drug Saf. 2003; 2:321–332.5. Bensouda-Grimaldi L, Jonville-Bera AP, Beau-Salinas F, Llabres S, Autret-Leca E. le reseau des centres regionaux de pharmacovigilance. Insertion problems, removal problems, and contraception failures with Implanon. Gynecol Obstet Fertil. 2005; 33:986–990.6. Implanon [Internet]. Kenilworth (NJ): Merck & Co.;c2011. cited 2017 Mar 13. Available from: http://www.implanon.com.7. Edwards JE, Moore A. Implanon: a review of clinical studies. Br J Fam Plann. 1999; 24:3–16.8. Heudes PM, Querat VL, Darnis E, Defrance C, Douane F, Frampas E. Migration of a contraceptive subcutaneous device into the pulmonary artery: report of a case. Case Rep Womens Health. 2015; 8:6–8.9. O'Brien A, O'Reilly MK, Sugrue G, Lawler L, Farrelly C. Subdermal contraceptive implant embolism to a pulmonary artery. Ann Thorac Surg. 2015; 99:2254–2255.10. James P, Trenery J. Ultrasound localisation and removal of non-palpable Implanon implants. Aust N Z J Obstet Gynaecol. 2006; 46:225–228.11. Diaz S, Pavez M, Miranda P, Robertson DN, Sivin I, Croxatto HB. A five-year clinical trial of levonorgestrel silastic implants (Norplant). Contraception. 1982; 25:447–456.12. Evans R, Holman R, Lindsay E. Migration of implanon: two case reports. J Fam Plann Reprod Health Care. 2005; 31:71–72.13. Organon Laboratories. Implanon: summary of product characteristics. place unknown: Organon Laboratories;2003.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Removal of migrated subdermal contraceptive implant (Implanon) to axillar: A case report
- Laparoscopic Removal of an Intrauterine Contraceptive Device That Had Migrated into the Intraabdominal Cavity
- Acute Median Neuropathy after Subdermal Contraceptive Implant Removal
- Bladder Stone Formation on Intra-uterine Contraceptive Device Perforated into the Bladder
- Intravesical Migration of an Intrauterine Contraceptive Device with Secondary Calculus Formation