Korean J Neurotrauma.
2015 Oct;11(2):135-138. 10.13004/kjnt.2015.11.2.135.
Lytic Complications after Skull Reconstruction Using GeneX(R)
- Affiliations
-
- 1Department of Neurosurgery, Daegu Fatima Hospital, Daegu, Korea. ns7012@daum.net
- KMID: 2378272
- DOI: http://doi.org/10.13004/kjnt.2015.11.2.135
Abstract
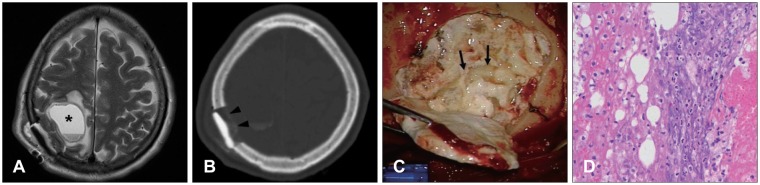
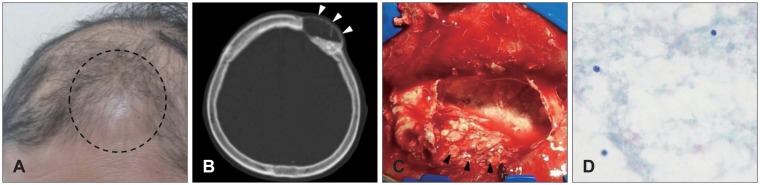
- Multiple methods and materials are available for bone defect reconstruction. Bone graft substitute is one of the materials used for reconstruction of bone defect and have been widely used recently. This report describes some cases about complications related to GeneX(R) which is introduced as mixture of calcium sulfate and beta-tricalcium phosphate at manufacturer's official web site. It informed of 3 patients who suffered wound inflammation, serous cyst after using GeneX(R) for reconstructing skull defect.
MeSH Terms
Figure
Reference
-
1. Friesenbichler J, Maurer-Ertl W, Sadoghi P, Pirker-Fruehauf U, Bodo K, Leithner A. Adverse reactions of artificial bone graft substitutes: lessons learned from using tricalcium phosphate geneX®. Clin Orthop Relat Res. 2014; 472:976–982. PMID: 24078171.
Article2. Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005; 36(Suppl 3):S20–S27. PMID: 16188545.
Article3. Johnson LJ, Clayer M. Aqueous calcium sulphate as bone graft for voids following open curettage of bone tumours. ANZ J Surg. 2013; 83:564–570. PMID: 22925525.
Article4. Kelly CM, Wilkins RM, Gitelis S, Hartjen C, Watson JT, Kim PT. The use of a surgical grade calcium sulfate as a bone graft substitute: results of a multicenter trial. Clin Orthop Relat Res. 2001; (382):42–50. PMID: 11154003.5. Lee GH, Khoury JG, Bell JE, Buckwalter JA. Adverse reactions to OsteoSet bone graft substitute, the incidence in a consecutive series. Iowa Orthop J. 2002; 22:35–38. PMID: 12180608.6. Robinson D, Alk D, Sandbank J, Farber R, Halperin N. Inflammatory reactions associated with a calcium sulfate bone substitute. Ann Transplant. 1999; 4:91–97. PMID: 10853791.7. Saadoun S, Macdonald C, Bell BA, Papadopoulos MC. Dangers of bone graft substitutes: lessons from using GeneX. J Neurol Neurosurg Psychiatry. 2011; 82:e3. PMID: 21386107.
Article8. Spetzger U, Vougioukas V, Schipper J. Materials and techniques for osseous skull reconstruction. Minim Invasive Ther Allied Technol. 2010; 19:110–121. PMID: 20166839.
Article9. Thomas MV, Puleo DA. Properties and clinical applications. J Biomed Mater Res B Appl Biomater. 2009; 88:597–610. PMID: 19025981.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cranial Fasciitis of Childhood: A case report
- Clinical & Radiological Evaluation of Skull Bone Tumors
- Usefulness of Synthetic Osteoconductive Bone Graft Substitute with Zeta Potential Control for Intramedullary Fixation with Proximal Femur Nail Antirotation in Osteoporotic Unstable Femoral Intertrochanteric Fracture
- Osteomyelitis of the skull
- Endoscopic Endonasal Skull Base Repair with Nasoseptal Flap