Infect Chemother.
2017 Mar;49(1):57-61. 10.3947/ic.2017.49.1.57.
Establishment of Experimental Murine Peritonitis Model with Hog Gastric Mucin for Carbapenem-Resistant Gram-Negative Bacteria
- Affiliations
-
- 1Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea. symonlee@catholic.ac.kr
- 2Division of Infectious Diseases, Department of Internal Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2375100
- DOI: http://doi.org/10.3947/ic.2017.49.1.57
Abstract
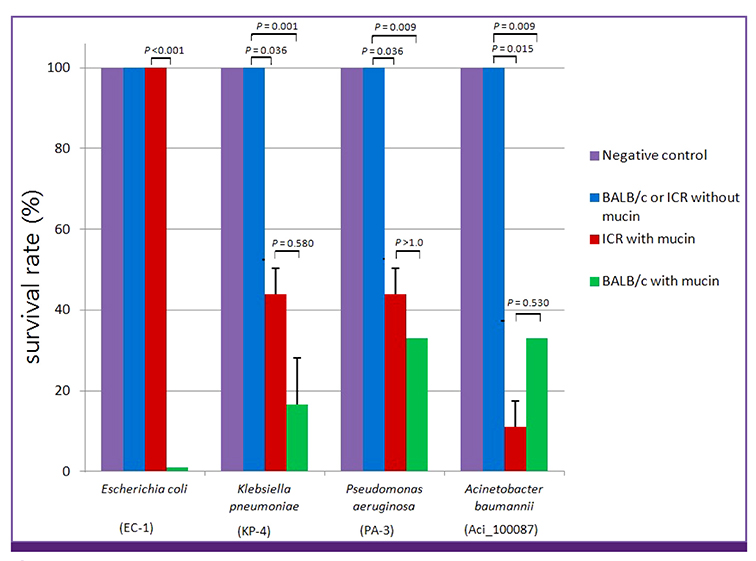
- Animal models are essential to studies of infectious diseases. The use of mice to test bacterial infection has been extensively reported. However, methods applied to clinical isolates, particularly for carbapenem-resistant bacteria, must be tailored according to the infection models and bacteria used. In this study, we infected 6-week-old female BALB/c mice intraperitoneally with different strains of resistant bacteria plus 3% hog gastric mucin. This method was found to be efficient and readily applicable for investigation of carbapenem-resisant Gram-negative pathogens (e.g., Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii) detected in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. World health organization (WHO). Global action plan on antimicrobial resistance. Accessed 14 October 2016. Available at: http://www.who.int/antimicrobial-resistance/global-action-plan/en/.2. Lee CS, Doi Y. Therapy of infections due to carbapenem-resistant Gram-negative pathogens. Infect Chemother. 2014; 46:149–164.
Article3. van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013; 75:115–120.
Article4. Lee HJ, Choi JK, Cho SY, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, Yoo JH. Carbapenem-resistant Enterobacteriaceae: prevalence and risk factors in a single community-based hospital in Korea. Infect Chemother. 2016; 48:166–173.
Article5. Pan CY, Chen JC, Chen TL, Wu JL, Hui CF, Chen JY. Piscidin is highly active against carbapenem-resistant Acinetobacter baumannii and NDM-1-producing Klebsiella pneumoniae in a systemic septicaemia infection mouse model. Mar Drugs. 2015; 13:2287–2305.
Article6. Cao F, Wang X, Wang L, Li Z, Che J, Wang L, Li X, Cao Z, Zhang J, Jin L, Xu Y. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. Biomed Res Int. 2015; 2015:752930.7. Aoki N, Tateba K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, Tanabe Y, Gejyo F, Yamaguchi K. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa . J Antimicrob Chemother. 2009; 63:534–542.
Article8. Pan CY, Chen JC, Sheen JF, Lin TL, Chen JY. Epinecidin-1 has immunomodulatory effects, facilitating its therapeutic use in a mouse model of Pseudomonas aeruginosa sepsis. Antimicrob Agents Chemother. 2014; 58:4264–4274.
Article9. Harris G, Kuo Lee R, Lam CK, Kanzaki G, Patel GB, Xu HH, Chen W. A mouse model of Acinetobacter baumannii–associated pneumonia using a clinically isolated hypervirulent strain. Antimicrob Agents Chemother. 2013; 57:3601–3613.
Article10. Rosen DA, Hilliard JK, Tiemann KM, Todd EM, Morley SC, Hunstad DA. Klebsiella pneumoniae FimK promotes virulence in murine pneumonia. J Infect Dis. 2016; 213:649–658.11. He S, He H, Chen Y, Wang W, Yu D. In vitro and in vivo analysis of antimicrobial agents alone and in combination against multi-drug resistant Acinetobacter baumannii . Front Microbiol. 2015; 6:507.12. Luo G, Spellberg B, Gebremariam T, Bolaris M, Lee H, Fu Y, French SW, Ibrahim AS. Diabetic murine models for Acinetobacter baumannii infection. J Antimicrob Chemother. 2012; 67:1439–1445.13. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: Twenty-fifth informational supplement M100-S25. Wayne, PA: CLSI;2015.14. Kim SH, Park C, Chun HS, Lee DG, Choi JK, Lee HJ, Cho SY, Park SH, Choi SM, Choi JH, Yoo JH. Pilot screening to determine antimicrobial synergies in a multidrug-resistant bacterial strain library. Microb Drug Resist. 2016; 22:372–378.
Article15. Division of laboratory animal resources (DLAR). Commonoly used mouse strains. Accessed 26 August 2016. Available at: https://www.research.uky.edu/dlar/documents/CommonMouseResearchModels.pdf.16. Olitzki L. Mucin as a resistance-lowering substance. Bacteriol Rev. 1948; 12:149–172.
Article17. Dewitt CW. Differential effect of hog gastric mucin on properdin and host resistance to infection. J Bacteriol. 1958; 76:631–639.
Article18. Rodriguez CA, Aqudelo M, Gonzalez JM, Vesga O, Zuluaga AF. An optimized mouse thigh infection model for enterococci and its impact on antimicrobial phamacodynamics. Antimicrob Agents Chemother. 2015; 59:233–238.
Article19. Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996; 64:1659–1665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antimicrobial Therapy for Infections Caused by Carbapenem-Resistant Gram-Negative Bacteria
- Calculation of Minimal Inhibitory Concentrations of Carbapenem from Zone Diameters of Disk Diffusion Test of Carbapenem-Resistant Nonfermentative Gram-Negative Bacilli
- Antibiotics for multidrug-resistant gram-negative bacteria
- Strategies to combat Gram-negative bacterial resistance to conventional antibacterial drugs: a review
- Carbapenem resistance in critically important human pathogens isolated from companion animals: a systematic literature review