Yonsei Med J.
2016 Jul;57(4):1038-1041. 10.3349/ymj.2016.57.4.1038.
The Effect of Xanthigen on the Expression of Brown Adipose Tissue Assessed by 18F-FDG PET
- Affiliations
-
- 1Department of Family Practice and Community Health, Ajou University School of Medicine, Suwon, Korea. jchcmc@hanmail.net
- 2Green Cross Reference Lab, Seoul, Korea.
- KMID: 2374141
- DOI: http://doi.org/10.3349/ymj.2016.57.4.1038
Abstract
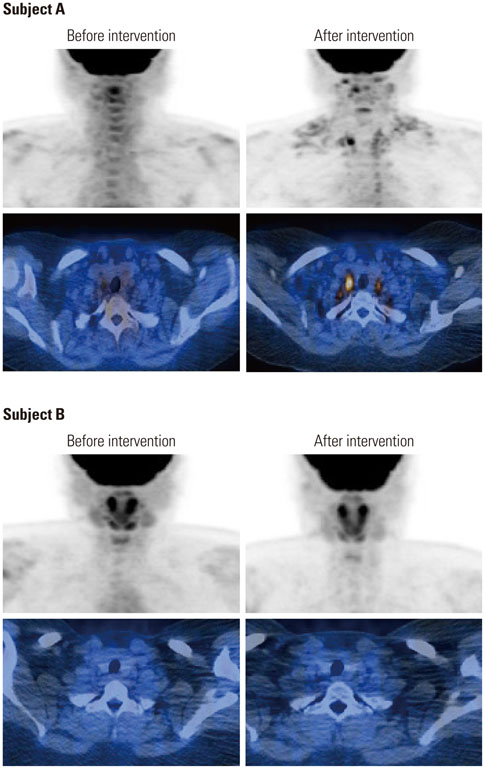
- Brown adipose tissue (BAT) is related with energy expenditure, in contrary to fat-storing white adipose tissue. Recent studies have shown that cold exposure could be related with the expression of BAT in adult subjects assessed by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET). In addition, the application in previous clinical trials showed positive effect of xanthigen containing fucoxanthin and punicic acid on body weight and liver fat content. In this short-term intervention study, we evaluated the effect of xanthigen on the expression of BAT by 18F-FDG PET. Two healthy obese premenopausal women were enrolled and xanthigen 600 mg (2 capsules including fucoxanthin 3 mg, punicic acid 174 mg) was given for 3 months without dietary and exercise intervention. Body composition and dietary intake were assessed monthly. Laboratory test and 18F-FDG PET were performed before and after intervention. After intervention, there was neither weight reduction nor remarkable laboratory change. However, BAT, assessed by 18F-FDG PET, was detected in both cervical, supraclavicular and paravertebral space in one subject, even though her body weight showed mild increase. This result suggested that xanthigen can induce BAT in a healthy adult. However, a further large well-controlled study is needed.
Keyword
Figure
Reference
-
1. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009; 360:1500–1508.
Article2. Yoneshiro T, Aita S, Matsushita M, Kameya T, Nakada K, Kawai Y, et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring). 2011; 19:13–16.
Article3. Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005; 332:392–397.
Article4. Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes Metab. 2010; 12:72–81.
Article5. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012; 150:366–376.
Article6. Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012; 7:e49452.
Article7. Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013; 495:379–383.
Article8. Broeders E, Bouvy ND, van Marken. Endogenous ways to stimulate brown adipose tissue in humans. Ann Med. 2015; 47:123–132.
Article9. Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, et al. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta. 2013; 1831:950–959.
Article10. Rossato M, Granzotto M, Macchi V, Porzionato A, Petrelli L, Calcagno A, et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol Cell Endocrinol. 2014; 383:137–146.
Article11. Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2013; 1831:969–985.
Article12. Hashimoto T, Ozaki Y, Taminato M, Das SK, Mizuno M, Yoshimura K, et al. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br J Nutr. 2009; 102:242–248.
Article13. Sangeetha RK, Bhaskar N, Divakar S, Baskaran V. Bioavailability and metabolism of fucoxanthin in rats: structural characterization of metabolites by LC-MS (APCI). Mol Cell Biochem. 2010; 333:299–310.
Article14. Maeda H. Nutraceutical effects of fucoxanthin for obesity and diabetes therapy: a review. J Oleo Sci. 2015; 64:125–132.
Article15. Gammone MA, D'Orazio N. Anti-obesity activity of the marine carotenoid fucoxanthin. Mar Drugs. 2015; 13:2196–2214.
Article16. Lee YH, Jung YS, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med. 2014; 46:e78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Perirenal 18F-FDG Uptake in a Patient with a Pheochromocytoma
- Evaluation of 18F-FDG Uptake Pattern in Brown Adipose Tissue Over Extended Time Period as Assessed by Multiple Time Point 18F-FDG-PET
- Esophageal Leiomyoma with intense FDG uptake on 18F-FDG PET/CT
- Application of Simultaneous 18F-FDG PET/MRI for Evaluating Residual Lesion in Pyogenic Spine Infection:a Case Report
- Use of 18F-FDG PET/CT in Second Primary Cancer