Yonsei Med J.
2016 May;57(3):754-760. 10.3349/ymj.2016.57.3.754.
The Role of Steroid Sulfatase as a Prognostic Factor in Patients with Endometrial Cancer
- Affiliations
-
- 1Division of Gynecologic Oncology and Gynecologic Minimally Invasive Surgery, Department of Obstetrics and Gynecology, Hanyang University College of Medicine, Seoul, Korea. obgybae@hanyang.ac.kr
- 2Department of Pathology, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2374098
- DOI: http://doi.org/10.3349/ymj.2016.57.3.754
Abstract
- PURPOSE
The aim of the study was to determine steroid sulfatase (STS) expression in endometrial cancer patients and its correlation with disease prognosis.
MATERIALS AND METHODS
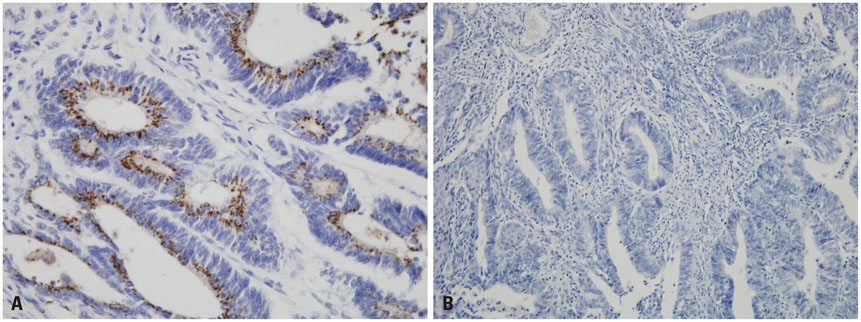
We conducted a retrospective study in 59 patients who underwent surgery with histologically confirmed endometrial cancer from January 2000 to December 2011 at Hanyang University Hospital. Immuno-histochemical staining of STS was performed using rabbit polyclonal anti-STS antibody.
RESULTS
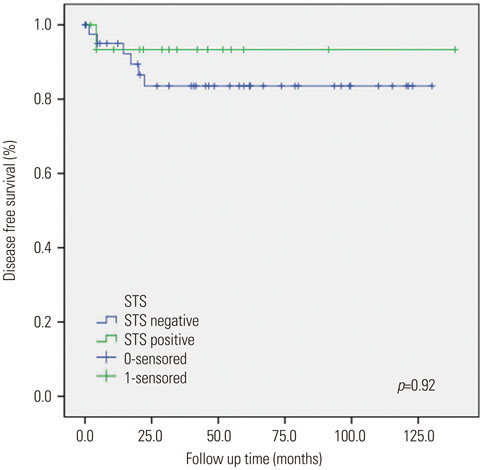
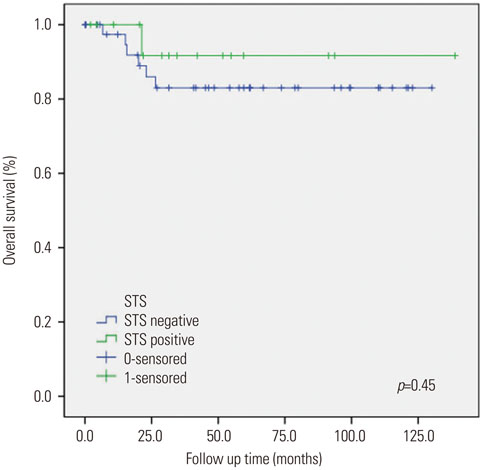
Sixteen of the 59 patients (27.1%) were positive for STS expression. Disease free survival (DFS) was 129.83±8.67 [95% confidence interval (CI): 112.84-146.82] months in the STS positive group (group A) and 111.06±7.17 (95% CI: 97.01-125.10) months in the STS negative group (group B) (p=0.92). Overall survival (OS) was 129.01±9.38 (95% CI: 110.63-147.38) months and 111.16±7.10 (95% CI: 97.24-125.07) months for the groups A and B, respectively (p=0.45). Univariate analysis revealed that FIGO stage and adjuvant therapy are significantly associated with DFS and OS. However, in multivariate analysis, FIGO stage and adjuvant therapy did not show any statistical significance with DFS and OS. STS was also not significantly associated with DFS and OS in univariate and multivariate analysis.
CONCLUSION
STS expression was not significantly associated with DFS and OS, despite positive STS expression in 27% of endometrial cancer patients. Therefore, the role of STS as a prognostic factor in patients with endometrial cancer remains unclear and requires further research.
MeSH Terms
-
Adult
Aged
Biomarkers, Tumor
Combined Modality Therapy
Disease-Free Survival
Endometrial Neoplasms/mortality/*surgery
Female
Gene Expression Regulation, Neoplastic
Humans
Middle Aged
Neoplasm Staging
Prognosis
Retrospective Studies
Steryl-Sulfatase/*metabolism
Uterine Neoplasms/mortality/pathology/*surgery
Biomarkers, Tumor
Steryl-Sulfatase
Figure
Reference
-
1. Sherman ME, Sturgeon S, Brinton L, Kurman RJ. Endometrial cancer chemoprevention: implications of diverse pathways of carcinogenesis. J Cell Biochem Suppl. 1995; 23:160–164.
Article2. Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Mod Pathol. 2000; 13:295–308.
Article3. Thomas DB. Do hormones cause breast cancer? Cancer. 1984; 53:3 Suppl. 595–604.
Article4. Kelsey JL, LiVolsi VA, Holford TR, Fischer DB, Mostow ED, Schwartz PE, et al. A case-control study of cancer of the endometrium. Am J Epidemiol. 1982; 116:333–342.
Article5. Ryan KJ. Editorial: Cancer risk and estrogen use in the menopause. N Engl J Med. 1975; 293:1199–1200.6. Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005; 26:171–202.
Article7. Woo LW, Purohit A, Potter BV. Development of steroid sulfatase inhibitors. Mol Cell Endocrinol. 2011; 340:175–185.
Article8. Geisler J, Sasano H, Chen S, Purohit A. Steroid sulfatase inhibitors: promising new tools for breast cancer therapy? J Steroid Biochem Mol Biol. 2011; 125:39–45.
Article9. Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995; 80:2918–2925.
Article10. Sasano H, Frost AR, Saitoh R, Harada N, Poutanen M, Vihko R, et al. Aromatase and 17 beta-hydroxysteroid dehydrogenase type 1 in human breast carcinoma. J Clin Endocrinol Metab. 1996; 81:4042–4046.
Article11. Utsunomiya H, Suzuki T, Kaneko C, Takeyama J, Nakamura J, Kimura K, et al. The analyses of 17beta-hydroxysteroid dehydrogenase isozymes in human endometrial hyperplasia and carcinoma. J Clin Endocrinol Metab. 2001; 86:3436–3443.
Article12. Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, et al. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987; 47:5616–5619.13. Utsunomiya H, Ito K, Suzuki T, Kitamura T, Kaneko C, Nakata T, et al. Steroid sulfatase and estrogen sulfotransferase in human endometrial carcinoma. Clin Cancer Res. 2004; 10:5850–5856.
Article14. Purohit A, Woo LW, Potter BV, Reed MJ. In vivo inhibition of estrone sulfatase activity and growth of nitrosomethylurea-induced mammary tumors by 667 COUMATE. Cancer Res. 2000; 60:3394–3396.15. Foster PA, Newman SP, Chander SK, Stengel C, Jhalli R, Woo LL, et al. In vivo efficacy of STX213, a second-generation steroid sulfatase inhibitor, for hormone-dependent breast cancer therapy. Clin Cancer Res. 2006; 12:5543–5549.
Article16. Foster PA, Chander SK, Parsons MF, Newman SP, Woo LW, Potter BV, et al. Efficacy of three potent steroid sulfatase inhibitors: pre-clinical investigations for their use in the treatment of hormone-dependent breast cancer. Breast Cancer Res Treat. 2008; 111:129–138.
Article17. Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, et al. Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res. 2006; 12:1585–1592.
Article18. Foster PA, Woo LW, Potter BV, Reed MJ, Purohit A. The use of steroid sulfatase inhibitors as a novel therapeutic strategy against hormone-dependent endometrial cancer. Endocrinology. 2008; 149:4035–4042.
Article19. Selcer KW, Difrancesca HM, Chandra AB, Li PK. Immunohistochemical analysis of steroid sulfatase in human tissues. J Steroid Biochem Mol Biol. 2007; 105:115–123.
Article20. Greggi S, Mangili G, Scaffa C, Scala F, Losito S, Iodice F, et al. Uterine papillary serous, clear cell, and poorly differentiated endometrioid carcinomas: a comparative study. Int J Gynecol Cancer. 2011; 21:661–667.
Article21. Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Haddock MG, et al. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecol Oncol. 2013; 129:478–485.
Article22. Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol. 2004; 94:256–266.
Article23. Neven P, Vergote I. Controversies regarding tamoxifen and uterine carcinoma. Curr Opin Obstet Gynecol. 1998; 10:9–14.
Article24. Slomovitz BM, Sun CC, Ramirez PT, Bodurka DC, Diaz P, Lu KH. Does tamoxifen use affect prognosis in breast cancer patients who develop endometrial cancer? Obstet Gynecol. 2004; 104:255–260.
Article25. DeMichele A, Troxel AB, Berlin JA, Weber AL, Bunin GR, Turzo E, et al. Impact of raloxifene or tamoxifen use on endometrial cancer risk: a population-based case-control study. J Clin Oncol. 2008; 26:4151–4159.
Article26. Berstein L, Maximov S, Gershfeld E, Meshkova I, Gamajunova V, Tsyrlina E, et al. Neoadjuvant therapy of endometrial cancer with the aromatase inhibitor letrozole: endocrine and clinical effects. Eur J Obstet Gynecol Reprod Biol. 2002; 105:161–165.
Article27. Barker LC, Brand IR, Crawford SM. Sustained effect of the aromatase inhibitors anastrozole and letrozole on endometrial thickness in patients with endometrial hyperplasia and endometrial carcinoma. Curr Med Res Opin. 2009; 25:1105–1109.
Article28. Ma BB, Oza A, Eisenhauer E, Stanimir G, Carey M, Chapman W, et al. The activity of letrozole in patients with advanced or recurrent endometrial cancer and correlation with biological markers--a study of the National Cancer Institute of Canada Clinical Trials Group. Int J Gynecol Cancer. 2004; 14:650–658.
Article29. Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol. 2000; 74:287–296.
Article30. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997; 138:863–870.
Article31. Bryant W, Snowhite AE, Rice LW, Shupnik MA. The estrogen receptor (ER)alpha variant Delta5 exhibits dominant positive activity on ER-regulated promoters in endometrial carcinoma cells. Endocrinology. 2005; 146:751–759.
Article32. Lin SL, Yan LY, Liang XW, Wang ZB, Wang ZY, Qiao J, et al. A novel variant of ER-alpha, ER-alpha36 mediates testosterone-stimulated ERK and Akt activation in endometrial cancer Hec1A cells. Reprod Biol Endocrinol. 2009; 7:102.
Article33. Ogawa S, Inoue S, Watanabe T, Orimo A, Hosoi T, Ouchi Y, et al. Molecular cloning and characterization of human estrogen receptor betacx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 1998; 26:3505–3512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis of x-linked ichthyosis and detection of its carriers with southern blot hybidization
- The prognostic significance of steroid hormone receptors, bcl-2 and p53 mutation in correlation with clinicopathological prognostic factors in endometrial cancer
- The role of lymphadenectomy in surgical staging of endometrial cancer
- Induction of Integrin Signaling by Steroid Sulfatase in Human Cervical Cancer Cells
- The prognostic value of serum CA 125 in endometrial cancer