Yonsei Med J.
2016 May;57(3):599-605. 10.3349/ymj.2016.57.3.599.
Lymphangiogenesis in Breast Cancer Correlates with Matrix Stiffness on Shear-Wave Elastography
- Affiliations
-
- 1Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. cho1988@yuhs.ac
- 2Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2374077
- DOI: http://doi.org/10.3349/ymj.2016.57.3.599
Abstract
- PURPOSE
To correlate tumor stiffness and lymphangiogenesis in breast cancer and to find its clinical implications.
MATERIALS AND METHODS
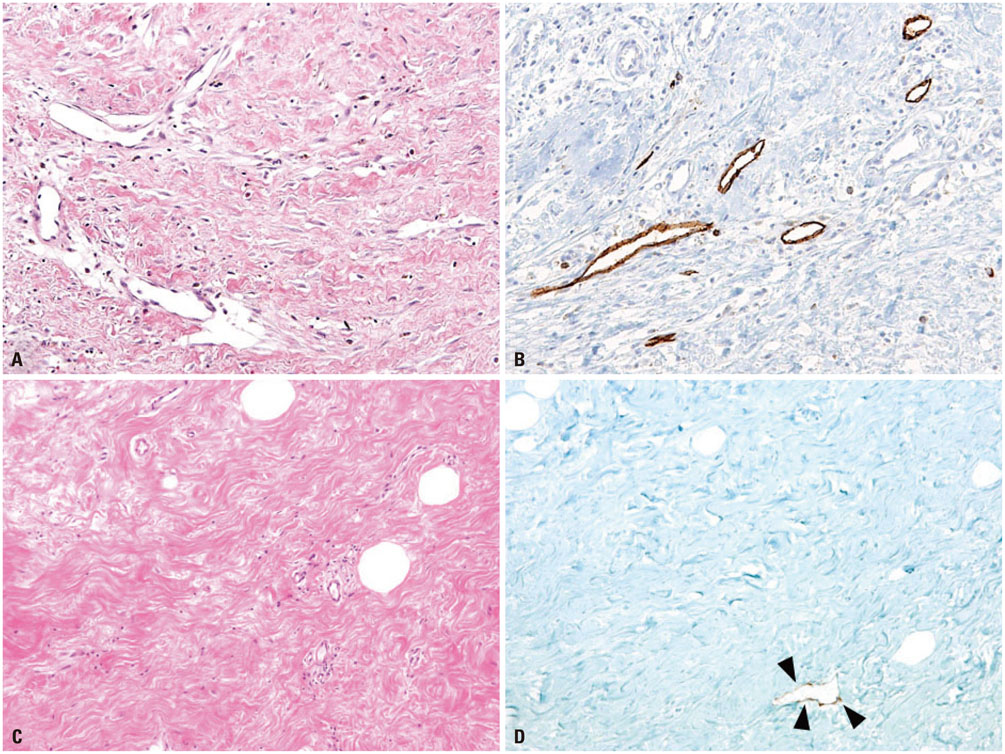
A total of 140 breast cancer patients were evaluated. Tumor stiffness was quantitatively measured by shear-wave elastography in preoperative ultrasound examination, calculated as mean elasticity value (kPa). Slides of resected breast cancer specimens were reviewed for most fibrotic area associated with tumor. D2-40 immunohistochemical staining was applied for fibrotic areas to detect the lymphatic spaces. Microlymphatic density, tumor stiffness, and clinicopathologic data were analyzed.
RESULTS
Higher elasticity value was associated with invasive size of tumor, microlymphatic density, histologic grade 3, absence of extensive intraductal component, presence of axillary lymph node metastasis, and Ki-67 labeling index (LI) in univariate regression analysis, and associated with Ki-67 LI and axillary lymph node metastasis in multivariate regression analysis. Microlymphatic density was associated histologic grade 3, mean elasticity value, and Ki-67 LI in univariate regression analysis. In multivariate regression analysis, microlymphatic density was correlated with mean elasticity value.
CONCLUSION
In breast cancer, tumor stiffness correlates with lymphangiogenesis and poor prognostic factors.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Breast/pathology
Breast Neoplasms/*diagnostic imaging/*pathology
Elasticity Imaging Techniques/*methods
Female
Humans
Lymph Nodes/pathology
Lymphangiogenesis/*physiology
Lymphatic Metastasis/*pathology
Middle Aged
Multivariate Analysis
Neoplasm Invasiveness
Neoplasm Staging
Regression Analysis
Figure
Reference
-
1. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006; 6:392–401.
Article2. Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998; 94:715–725.
Article3. Liu L, Zhu XD, Wang WQ, Shen Y, Qin Y, Ren ZG, et al. Activation of beta-catenin by hypoxia in hepatocellular carcinoma contributes to enhanced metastatic potential and poor prognosis. Clin Cancer Res. 2010; 16:2740–2750.
Article4. Zhang Q, Bai X, Chen W, Ma T, Hu Q, Liang C, et al. Wnt/β-catenin signaling enhances hypoxia-induced epithelial-mesenchymal transition in hepatocellular carcinoma via crosstalk with hif-1α signaling. Carcinogenesis. 2013; 34:962–973.
Article5. Van den Eynden GG, Colpaert CG, Couvelard A, Pezzella F, Dirix LY, Vermeulen PB, et al. A fibrotic focus is a prognostic factor and a surrogate marker for hypoxia and (lymph)angiogenesis in breast cancer: review of the literature and proposal on the criteria of evaluation. Histopathology. 2007; 51:440–451.
Article6. Van der Auwera I, Van den Eynden GG, Colpaert CG, Van Laere SJ, van Dam P, Van Marck EA, et al. Tumor lymphangiogenesis in inflammatory breast carcinoma: a histomorphometric study. Clin Cancer Res. 2005; 11:7637–7642.
Article7. Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002; 2:38–47.8. Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004; 9:Suppl 5. 10–17.
Article9. Hwang JY, Han BK, Ko EY, Shin JH, Hahn SY, Nam MY. Screening ultrasound in women with negative mammography: outcome analysis. Yonsei Med J. 2015; 56:1352–1358.
Article10. Chang JM, Park IA, Lee SH, Kim WH, Bae MS, Koo HR, et al. Stiffness of tumours measured by shear-wave elastography correlated with subtypes of breast cancer. Eur Radiol. 2013; 23:2450–2458.
Article11. Evans A, Whelehan P, Thomson K, McLean D, Brauer K, Purdie C, et al. Invasive breast cancer: relationship between shear-wave elastographic findings and histologic prognostic factors. Radiology. 2012; 263:673–677.
Article12. Pallwein L, Aigner F, Faschingbauer R, Pallwein E, Pinggera G, Bartsch G, et al. Prostate cancer diagnosis: value of real-time elastography. Abdom Imaging. 2008; 33:729–735.
Article13. Pozzi E, Mantica G, Gastaldi C, Berardinelli M, Choussos D, Bianchi CM, et al. The role of the elastography in the diagnosis of prostate cancer: a retrospective study on 460 patients. Arch Ital Urol Androl. 2012; 84:151–154.14. Corpechot C, Carrat F, Poujol-Robert A, Gaouar F, Wendum D, Chazouillères O, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology. 2012; 56:198–208.
Article15. Roca B, Resino E, Torres V, Herrero E, Penades M. Interobserver discrepancy in liver fibrosis using transient elastography. J Viral Hepat. 2012; 19:711–715.
Article16. Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, et al. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006; 95:1611–1625.
Article17. Jitsuiki Y, Hasebe T, Tsuda H, Imoto S, Tsubono Y, Sasaki S, et al. Optimizing microvessel counts according to tumor zone in invasive ductal carcinoma of the breast. Mod Pathol. 1999; 12:492–498.18. Lee J, Park S, Kim S, Kim J, Ryu J, Park HS, et al. Characteristics and survival of breast cancer patients with multiple synchronous or metachronous primary cancers. Yonsei Med J. 2015; 56:1213–1220.
Article19. Youk JH, Gweon HM, Son EJ, Kim JA, Jeong J. Shear-wave elastography of invasive breast cancer: correlation between quantitative mean elasticity value and immunohistochemical profile. Breast Cancer Res Treat. 2013; 138:119–126.
Article20. Choi WJ, Kim HH, Cha JH, Shin HJ, Kim H, Chae EY, et al. Predicting prognostic factors of breast cancer using shear wave elastography. Ultrasound Med Biol. 2014; 40:269–274.
Article21. Evans A, Rauchhaus P, Whelehan P, Thomson K, Purdie CA, Jordan LB, et al. Does shear wave ultrasound independently predict axillary lymph node metastasis in women with invasive breast cancer? Breast Cancer Res Treat. 2014; 143:153–157.
Article22. Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013; 139:539–552.
Article23. Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer. 2009; 9:108–122.
Article24. Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009; 139:891–906.
Article25. Colpaert C, Vermeulen P, van Beest P, Goovaerts G, Weyler J, Van Dam P, et al. Intratumoral hypoxia resulting in the presence of a fibrotic focus is an independent predictor of early distant relapse in lymph node-negative breast cancer patients. Histopathology. 2001; 39:416–425.
Article26. Kornegoor R, Verschuur-Maes AH, Buerger H, Hogenes MC, de Bruin PC, Oudejans JJ, et al. Fibrotic focus and hypoxia in male breast cancer. Mod Pathol. 2012; 25:1397–1404.
Article27. Colpaert CG, Vermeulen PB, Fox SB, Harris AL, Dirix LY, Van Marck EA. The presence of a fibrotic focus in invasive breast carcinoma correlates with the expression of carbonic anhydrase IX and is a marker of hypoxia and poor prognosis. Breast Cancer Res Treat. 2003; 81:137–147.
Article28. Van den, Smid M, Van Laere SJ, Colpaert CG, Van der Auwera I, Bich TX, et al. Gene expression profiles associated with the presence of a fibrotic focus and the growth pattern in lymph node-negative breast cancer. Clin Cancer Res. 2008; 14:2944–2952.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Performance of Quantitative Shear Wave Ultrasound Elastography for Thyroid Cancer
- Future of breast elastography
- Shear-wave elastography in breast ultrasonography: the state of the art
- Ultrasound Elastography for Liver Disease with Focus on Hepatic Fibrosis
- Age-related change in shear elastic modulus of the thoracolumbar multifidus muscle in healthy Beagle dogs using ultrasound shear wave elastography