J Korean Med Sci.
2016 Jun;31(6):873-878. 10.3346/jkms.2016.31.6.873.
Clinicopathologic Similarities of the Main and Minor Lesions of Synchronous Multiple Early Gastric Cancer
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea. pdk66@gilhospital.com
- 2Depatment of Internal Medicine, School of Medicine, Gachon University, Incheon, Korea.
- 3Gachon Medical Research Institute, Gachon University Gil Medical Center, Incheon, Korea.
- 4Department of Surgery, Gachon University Gil Medical Center, Gachon University School of Medicine, Incheon, Korea.
- 5Department of Pathology, Gachon University Gil Medical Center, Gachon University School of Medicine, Incheon, Korea.
- KMID: 2373695
- DOI: http://doi.org/10.3346/jkms.2016.31.6.873
Abstract
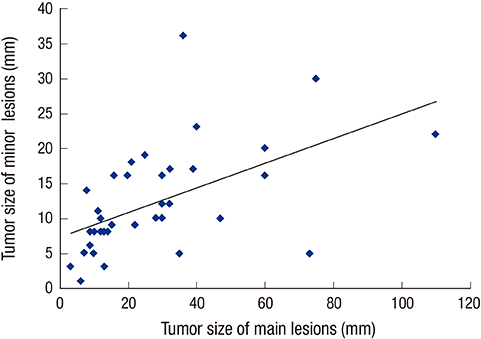
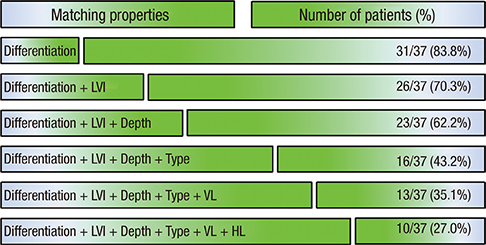
- The detection rate of early gastric cancer (EGC) is increasing due to improvements in diagnostic methods, but synchronous multiple EGC (SMEGC) remains a major problem. Therefore, we investigated the characteristics of and the correlation between the main and minor lesions of SMEGC. We retrospectively reviewed the medical records of patients with EGC between April 2008 and May 2013. The main lesion was defined as the one with the greatest invasion depth. If lesions had the same invasion depth, the tumor diameter was used to define the main lesion. Of 963 patients who had treatment for EGC, 37 patients with SMEGC were analyzed. The main and minor lesions showed a significant positive correlation of size (r = 0.533, P = 0.001). The main and minor lesions of SMEGC showed the same vertical and horizontal locations at 70.3% and 64.9%, respectively (P = 0.002 and P = 0.002). Macroscopic types were identical in 67.6% (P < 0.001), and 32.4% had identical macroscopic type and location. The main and minor lesions had identical characteristics of invasion depth, presence of lymphovascular invasion (LVI), and differentiation in 78.4%, 83.8%, and 83.8%, respectively. Differentiation, LVI, and invasion depth (microscopic characteristics) were simultaneously the same in 62.2%. The location, macroscopic type, and 3 microscopic characteristics were matched in 27%. The main and minor lesions of SMEGC have similar clinicopathologic characteristics. Therefore, the possibility of SMEGC should not be neglected in cases of EGC, considering an understanding of the characteristics and association of lesions.
MeSH Terms
Figure
Reference
-
1. Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005; 37:990–993.2. Maehara Y, Kakeji Y, Oda S, Baba H, Sugimachi K. Tumor growth patterns and biological characteristics of early gastric carcinoma. Oncology. 2001; 61:102–112.3. Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, et al. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013; 62:1425–1432.4. Otsuji E, Kuriu Y, Ichikawa D, Okamoto K, Hagiwara A, Yamagishi H. Clinicopathologic characteristics and prognosis of synchronous multifocal gastric carcinomas. Am J Surg. 2005; 189:116–119.5. Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006; 9:93–98.6. Peng J, Wang Y. Epidemiology, pathology and clinical management of multiple gastric cancers: a mini-review. Surg Oncol. 2010; 19:e110–4.7. Nitta T, Egashira Y, Akutagawa H, Edagawa G, Kurisu Y, Nomura E, Tanigawa N, Shibayama Y. Study of clinicopathological factors associated with the occurrence of synchronous multiple gastric carcinomas. Gastric Cancer. 2009; 12:23–30.8. Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ. Helicobacter pylori eradication for prevention of metachronous recurrence after endoscopic resection of early gastric cancer. J Korean Med Sci. 2015; 30:749–756.9. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011; 14:101–112.10. Moertel CG, Bargen JA, Soule EH. Multiple gastric cancers; review of the literature and study of 42 cases. Gastroenterology. 1957; 32:1095–1103.11. Kim JH, Kim YJ, An J, Lee JJ, Cho JH, Kim KO, Chung JW, Kwon KA, Park DK, Kim JH. Endoscopic features suggesting gastric cancer in biopsy-proven gastric adenoma with high-grade neoplasia. World J Gastroenterol. 2014; 20:12233–12240.12. Kang KJ, Kim KM, Min BH, Lee JH, Kim JJ. Endoscopic submucosal dissection of early gastric cancer. Gut Liver. 2011; 5:418–426.13. Seo JH, Park JC, Kim YJ, Shin SK, Lee YC, Lee SK. Undifferentiated histology after endoscopic resection may predict synchronous and metachronous occurrence of early gastric cancer. Digestion. 2010; 81:35–42.14. Isobe T, Hashimoto K, Kizaki J, Murakami N, Aoyagi K, Koufuji K, Akagi Y, Shirouzu K. Characteristics and prognosis of synchronous multiple early gastric cancer. World J Gastroenterol. 2013; 19:7154–7159.15. Choi J, Kim SG, Im JP, Kang SJ, Lee HJ, Yang HK, Kim JS, Kim WH, Jung HC, Song IS. Lymph node metastasis in multiple synchronous early gastric cancer. Gastrointest Endosc. 2011; 74:276–284.16. Kim HM, Kim HK, Lee SK, Cho JH, Pak KH, Hyung WJ, Noh SH, Kim CB, Lee YC, Song SY, et al. Multifocality in early gastric cancer does not increase the risk of lymph node metastasis in a single-center study. Ann Surg Oncol. 2012; 19:1251–1256.17. Kitamura K, Yamaguchi T, Okamoto K, Otsuji E, Taniguchi H, Hagiwara A, Sawai K, Takahashi T. Clinicopathologic features of synchronous multifocal early gastric cancers. Anticancer Res. 1997; 17:643–646.18. Tan IB, Ivanova T, Lim KH, Ong CW, Deng N, Lee J, Tan SH, Wu J, Lee MH, Ooi CH, et al. Intrinsic subtypes of gastric cancer, based on gene expression pattern, predict survival and respond differently to chemotherapy. Gastroenterology. 2011; 141:476–485. 485.e1–485.e11.19. Ryu JW, Kim HJ, Lee YS, Myong NH, Hwang CH, Lee GS, Yom HC. The proteomics approach to find biomarkers in gastric cancer. J Korean Med Sci. 2003; 18:505–509.20. Ribeiro U Jr, Jorge UM, Safatle-Ribeiro AV, Yagi OK, Scapulatempo C, Perez RO, Corbett CE, Alves VA, Zilberstein B, Gama-Rodrigues J. Clinicopathologic and immunohistochemistry characterization of synchronous multiple primary gastric adenocarcinoma. J Gastrointest Surg. 2007; 11:233–239.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multiple Early Gastric Cancer
- The Clinicopathologic Features and Prognosis of Multiple Early Gastric Cancer
- A Case of Synchronous Triple Early Gastric Cancer
- Clinicopathologic Features of Multiple Synchronous Gastric Cancer
- Microsatellite Instability Is Associated with the Clinicopathologic Features of Gastric Cancer in Sporadic Gastric Cancer Patients