Ann Lab Med.
2016 Nov;36(6):550-554. 10.3343/alm.2016.36.6.550.
Evaluation of the VIDAS Anti-HCV Assay for Detection of Hepatitis C Virus Infection
- Affiliations
-
- 1Department of Laboratory Medicine, Hallym University College of Medicine, Hwaseong, Korea. hskim0901@empas.com
- 2Department of Laboratory Medicine, Inje University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea. chayoung@cau.ac.kr
- KMID: 2373593
- DOI: http://doi.org/10.3343/alm.2016.36.6.550
Abstract
- BACKGROUND
Anti-hepatitis C virus antibody (anti-HCV) assays are recommended for screening HCV-infected persons. The VIDAS Anti-HCV Assay (bioMérieux, France), based on the enzyme-linked fluorescence test principle, was recently introduced in Korea. We evaluated the clinical performance of the VIDAS assay.
METHODS
One hundred HCV-positive and 1,002 HCV-negative blood samples confirmed by Architect anti-HCV (Abbott Laboratories, USA) and COBAS TaqMan HCV real-time PCR (Roche Diagnostics, USA) or the Procleix Ultrio Plus Assay (Gen-Probe Incorporated, USA) were obtained from the Human Serum Bank (HSB) and tested by VIDAS. In case of discrepant results, we conducted a recombinant immunoblot assay (RIBA).
RESULTS
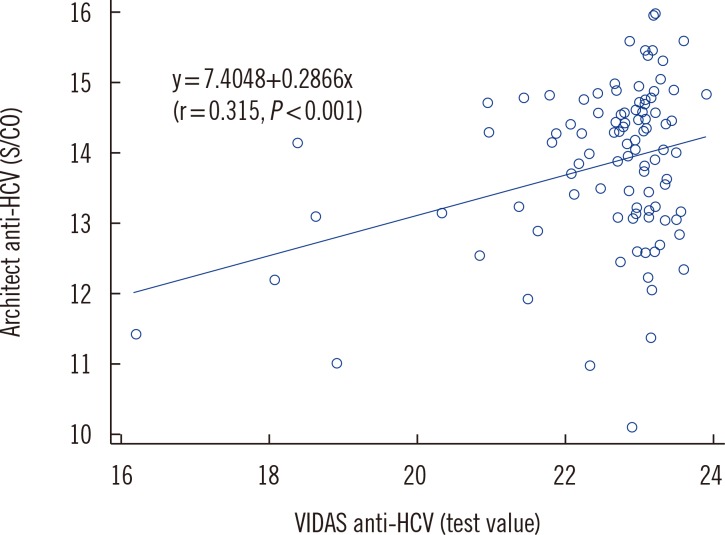
The agreement rates for known HCV-positive and HCV-negative samples between the VIDAS assay and the HSB testing were 100% (95% confidence interval [CI]: 96.4-100%) and 99.5% (95% CI: 98.8-99.8%), respectively. One of the five discrepant samples was positive for Core 2+ and NS3-2 2+ reactivity, two samples were negative, and the other two were indeterminate regarding NS4 2+ reactivity in RIBA. We observed a significant but weak positive correlation between the titers of VIDAS and Architect assays (r=0.315, P<0.001).
CONCLUSIONS
The VIDAS anti-HCV assay, developed on the VIDAS automated immunoassay platform based on the ready-to-use, single-sample test concept may be useful in small-to-medium-sized laboratories. It showed good agreement with Architect anti-HCV and COBAS PCR assays and is therefore useful for detection of HCV infection. Weakly test-positive (ambiguous) samples require additional testing by another anti-HCV, RIBA, or HCV RNA assay.
Keyword
MeSH Terms
Figure
Reference
-
1. WHO. Hepatitis C. Fact sheet No. 164. Updated on Jul 2015. http://www.who.int/mediacentre/factsheets/fs164/en.2. WHO. Guidelines for the screening care and treatment of persons with hepatitis c infection. WHO Press;2014. p. 1–222. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/.3. European Association for Study of L. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014; 60:392–420. PMID: 24331294.4. Kamili S, Drobeniuc J, Araujo AC, Hayden TM. Laboratory diagnostics for hepatitis C virus infection. Clin Infect Dis. 2012; 55(Sl):S43–S48. PMID: 22715213.5. Seignères B, Descamps F, Croise R, Barlet V, Bouvier-Alias M, Chevaliez S, et al. Multicenter clinical evaluation of the new 3rd generation assay for detection of antibodies against hepatitis C virus on the VIDAS system. J Clin Virol. 2016; 78:20–26. PMID: 26962723.6. Alborino F, Burighel A, Tiller FW, van Helden J, Gabriel C, Raineri A, et al. Multicenter evaluation of a fully automated third-generation anti-HCV antibody screening test with excellent sensitivity and specificity. Med Microbiol Immunol. 2011; 200:77–83. PMID: 20865278.7. Kim S, Kim JH, Yoon S, Park YH, Kim HS. Clinical performance evaluation of four automated chemiluminescence immunoassays for hepatitis C virus antibody detection. J Clin Microbiol. 2008; 46:3919–3923. PMID: 18945839.8. Park Y, Seok Y, Choi J, Kim HS. Performance evaluation of the Vitros anti-hepatitis C virus antibody assay for use in clinical laboratories. Clin Biochem. 2012; 45:175–177. PMID: 22093968.9. Watterson JM, Stallcup P, Escamilla D, Chernay P, Reyes A, Trevino SC. Evaluation of the Ortho-Clinical Diagnostics Vitros ECi Anti-HCV test: comparison with three other methods. J Clin Lab Anal. 2007; 21:162–166. PMID: 17506481.10. Berger A, Rabenau H, Allwinn R, Doerr HW. Evaluation of the new ARCHITECT anti-HCV screening test under routine laboratory conditions. J Clin Virol. 2008; 43:158–161. PMID: 18635393.11. Kiely P, Kay D, Parker S, Piscitelli L. The significance of third-generation HCV RIBA-indeterminate, RNA-negative results in voluntary blood donors screened with sequential third-generation immunoassays. Transfusion. 2004; 44:349–358. PMID: 14996191.12. Lemaire JM, Courouce AM, Defer C, Bouchardeau F, Coste J, Agulles O, et al. HCV RNA in blood donors with isolated reactivities by third-generation RIBA. Transfusion. 2000; 40:867–870. PMID: 10924617.13. Pawlotsky JM, Bastie A, Pellet C, Remire J, Darthuy F, Wolfe L, et al. Significance of indeterminate third-generation hepatitis C virus recombinant immunoblot assay. J Clin Microbiol. 1996; 34:80–83. PMID: 8748278.14. Alter MJ, Kuhnert WL, Finelli L. Centers for Disease Control and Prevention. Guidelines for laboratory testing and result reporting of antibody to hepatitis C virus. Centers for Disease Control and Prevention. MMWR Recomm Rep. 2003; 52:1–13. 15quiz CE1-4. PMID: 12585742.15. Centers for Disease Control and Prevention. Testing for HCV infection: an update of guidance for clinicians and laboratorians. MMWR Morb Mortal Wkly Rep. 2013; 62:362–365. PMID: 23657112.16. Yang R, Guan W, Wang Q, Liu Y, Wei L. Performance evaluation and comparison of the newly developed Elecsys anti-HCV II assay with other widely used assays. Clin Chim Acta. 2013; 426:95–101. PMID: 24056023.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Test Parameters on the Performance of Rapid Anti-Hepatitis C Virus Immunochromatographic Assay
- Usefulness of HCV Core Protein for Detection of HCV Viremia
- Evaluation of Genedia HCV Rapid for Rapid Detection of Antibody to Hepatitis C Virus
- Hepatitis C Virus Genotypes in Korean Hemophiliacs
- Laboratory Evaluation of the LG HCD 3.0TMB (LG HCD3.0 plus) for the Detection of HCV Antibodies