Ann Lab Med.
2016 Jan;36(1):15-22. 10.3343/alm.2016.36.1.15.
Rapid Detection of Pseudomonas aeruginosa and Acinetobacter baumannii Harboring blaVIM-2, blaIMP-1 and blaOXA-23 Genes by Using Loop-Mediated Isothermal Amplification Methods
- Affiliations
-
- 1Department of Microbiology and Immunology, Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. sangsun_yoon@yuhs.ac
- 2Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. deyong@yuhs.ac
- 3Institute for Immunology and Immunological Diseases, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2373492
- DOI: http://doi.org/10.3343/alm.2016.36.1.15
Abstract
- BACKGROUND
Carbapenem-resistant Pseudomonas aeruginosa (CRPA) and Acinetobacter baumannii (CRAB) are the leading causes of nosocomial infections. A rapid and sensitive test to detect CRPA and CRAB is required for appropriate antibiotic treatment. We optimized a loop-mediated isothermal amplification (LAMP) assay to detect the presence of bla(VIM-2), bla(IMP-1), and bla OXA-23, which are critical components for carbapenem resistance.
METHODS
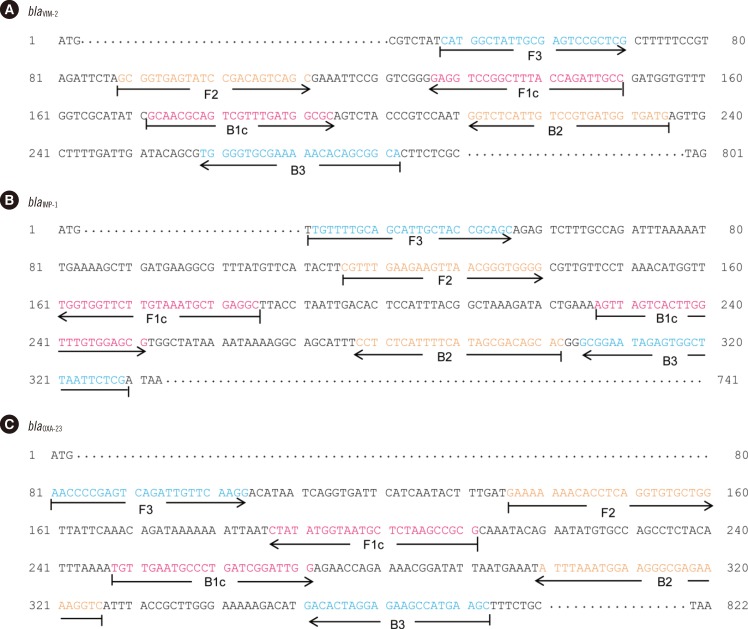
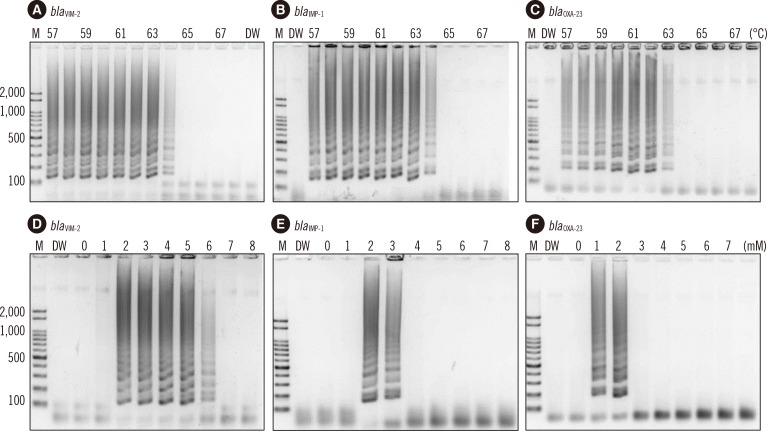
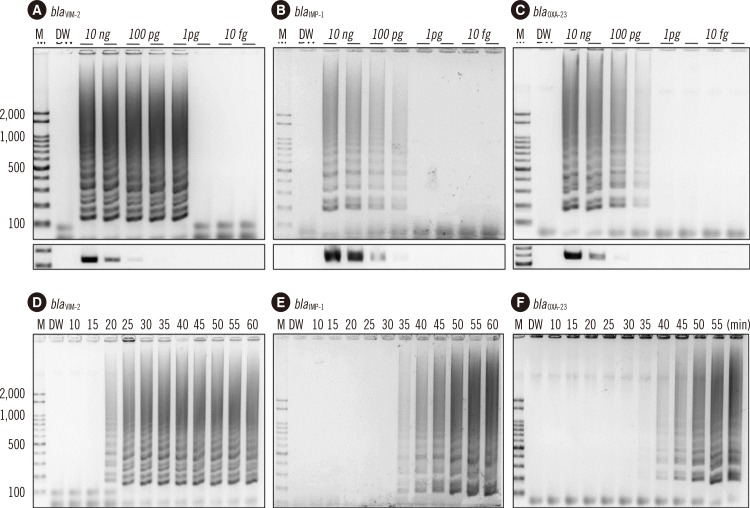
Two sets of primers, inner and outer primers, were manually designed as previously described. The LAMP buffer was optimized (at 2mM MgSO4) by testing different concentrations of MgSO4. The optimal reaction temperature and incubation time were determined by using a gradient thermocycler. Then, the optimized bla(VIM-2), bla(IMP-1), and bla(OXA-23) LAMP reactions were evaluated by using 120 P. aeruginosa and 99 A. baumannii clinical isolates.
RESULTS
Only one strain of the 100 CRPA isolates harbored bla(IMP-1), whereas none of them harbored bla(VIM-2). These results indicate that the acquisition of bla(VIM-2) or bla(IMP-1) may not play a major role in carbapenem resistance in Korea. Fifty two strains of the 75 CRAB isolates contained bla(OXA-23), but none contained bla(VIM-2) and bla(IMP-1) alleles.
CONCLUSIONS
Our results demonstrate the usefulness of LAMP for the diagnosis of CRPA and CRAB.
Keyword
MeSH Terms
-
Acinetobacter baumannii/genetics/*isolation & purification
Anti-Bacterial Agents/*pharmacology
Carbapenems/*pharmacology
Drug Resistance, Bacterial/*genetics
*Genes, Bacterial
Nucleic Acid Amplification Techniques
Pseudomonas aeruginosa/genetics/*isolation & purification
Sensitivity and Specificity
Anti-Bacterial Agents
Carbapenems
Figure
Reference
-
1. Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect. 2006; 12:826–836. PMID: 16882287.
Article2. Sevillano E, Valderrey C, Canduela MJ, Umaran A, Calvo F, Gallego L. Resistance to antibiotics in clinical isolates of Pseudomonas aeruginosa. Pathol Biol (Paris). 2006; 54:493–497. PMID: 17027190.3. Oh EJ, Lee S, Park YJ, Park JJ, Park K, Kim SI, et al. Prevalence of metallo-beta-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii in a Korean university hospital and comparison of screening methods for detecting metallo-beta-lactamase. J Microbiol Methods. 2003; 54:411–418. PMID: 12842488.4. Jeong SH, Bae IK, Park KO, An YJ, Sohn SG, Jang SJ, et al. Outbreaks of imipenem-resistant Acinetobacter baumannii producing carbapenemases in Korea. J Microbiol. 2006; 44:423–431. PMID: 16953178.5. Lee K, Park AJ, Kim MY, Lee HJ, Cho JH, Kang JO, et al. Metallo-beta-lactamase-producing Pseudomonas spp. in Korea: high prevalence of isolates with VIM-2 type and emergence of isolates with IMP-1 type. Yonsei Med J. 2009; 50:335–339. PMID: 19568593.6. Yong D, Choi YS, Roh KH, Kim CK, Park YH, Yum JH, et al. Increasing prevalence and diversity of metallo-beta-lactamases in Pseudomonas spp., Acinetobacter spp., and Enterobacteriaceae from Korea. Antimicrob Agents Chemother. 2006; 50:1884–1886. PMID: 16641469.7. Sung JY, Koo SH, Cho HH, Kwon KC. Dissemination of an AbaR-type resistance island in multidrug-resistant Acinetobacter baumannii global clone 2 in Daejeon of Korea. Ann Clin Microbiol. 2013; 16:75–80.8. Chung H-S, Lee Y, Park ES, Lee DS, Ha EJ, Kim M, et al. Characterization of the multidrug-resistant Acinetobacter species causing a nosocomial outbreak at intensive care units in a Korean teaching hospital: suggesting the correlations with the clinical and environmental samples, including respiratory tract-related instruments. Ann Clin Microbiol. 2014; 17:29–34.9. Lee J. Status on sentinel surveillance system of multidrug-resistant organisms in Korea. Updated in 2012. http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&cid=12713.10. Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007; 20:440–458. PMID: 17630334.11. Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001; 7:88–91. PMID: 11298149.12. Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003; 41:4623–4629. PMID: 14532193.13. Amjad A, Mirza I, Abbasi S, Farwa U, Malik N, Zia F. Modified Hodge test: A simple and effective test for detection of carbapenemase production. Iran J Microbiol. 2011; 3:189–193. PMID: 22530087.14. Markelz AE, Mende K, Murray CK, Yu X, Zera WC, Hospenthal DR, et al. Carbapenem susceptibility testing errors using three automated systems, disk diffusion, Etest, and broth microdilution and carbapenem resistance genes in isolates of Acinetobacter baumannii-calcoaceticus complex. Antimicrob Agents Chemother. 2011; 55:4707–4711. PMID: 21807971.
Article15. Kim HJ, Kim YJ, Yong DE, Lee K, Park JH, Lee JM, et al. Loop-mediated isothermal amplification of vanA gene enables a rapid and naked-eye detection of vancomycin-resistant enterococci infection. J Microbiol Methods. 2014; 104:61–66. PMID: 24925601.
Article16. Labarca JA, Salles MJ, Seas C, Guzmán-Blanco M. Carbapenem resistance in Pseudomonas aeruginosa and Acinetobacter baumannii in the nosocomial setting in Latin America. Crit Rev Microbiol. 2014; 1–17. PMID: 25159043.17. Kim BM, Jeon EJ, Jang JY, Chung JW, Park J, Choi JC, et al. Four year trend of carbapenem-resistance in newly opened ICUs of a university-affiliated hospital of South Korea. Tuberc Respir Dis (Seoul). 2012; 72:360–366. PMID: 23227077.
Article18. Tripathi A, Shukla SK, Singh A, Prasad KN. A new approach of real time polymerase chain reaction in detection of vancomycin-resistant enterococci and its comparison with other methods. Indian J Med Microbiol. 2013; 31:47–52. PMID: 23508429.
Article19. Kojima T, Shibata N, Ikedo M, Arakawa Y. Development and evaluation of novel loop-mediated isothermal amplification for rapid detection of bla(IMP-1) and bla(VIM-2) genes. Kansenshogaku Zasshi. 2006; 80:405–412. PMID: 16922484.20. Lee Y, Kim C-K, Chung H-S, Yong D, Jeong SH, Lee K, et al. Increasing carbapenem-resistant gram-negative bacilli and decreasing metallo-β-lactamase producers over eight years from Korea. Yonsei Med J. 2015; 56:572–577. PMID: 25684011.
Article21. Rodríguez-Martínez JM, Poirel L, Nordmann P. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009; 53:4783–4788. PMID: 19738025.22. Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2006; 50:1633–1641. PMID: 16641429.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence of Carbapenemase and Integrase Genes in Imipenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa Collected over Several Years in a University Hospital
- Prevalence of Metallo-beta-lactamases in Imipenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii
- In vitro Susceptibility of Imipenem-resistant Pseudomonas aeruginosa against Piperacillin/tazobactam
- Loop-mediated isothermal amplification assay for the detection of Salmonella spp. in pig feces
- Characterization of Class 1 Integrons in Metallo-beta-lactamase-producing Pseudomonas aeruginosa