Korean J Gastroenterol.
2015 Apr;65(4):246-251. 10.4166/kjg.2015.65.4.246.
Sorafenib in the Treatment of Recurrent Hepatocellular Carcinoma after Liver Transplantation: A Report of Four Cases
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea. ktyoon@pusan.ac.kr
- 2Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- 3Department of Internal Medicine, Pusan National University School of Medicine, Yangsan, Korea.
- 4Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea.
- KMID: 2373217
- DOI: http://doi.org/10.4166/kjg.2015.65.4.246
Abstract
- With an increasing number of patients with hepatocellular carcinoma (HCC) undergoing liver transplantation (LT), tumor recurrence remains the main limiting factor for long-term survival. Although sorafenib is available for advanced HCC, there is still a lack of data on the use of sorafenib for treatment of recurrent HCC after LT. Here, we report on four cases of the use of sorafenib for treatment of recurrent HCC after LT. The median time of recurrence from LT was 4 months (range, 1-16 months). Two of the four evaluated patients showed stable disease, which was the best response and the duration of stabilization was 11 months and 5 months, respectively. One patient also experienced stable disease and remained in stable disease without sorafenib therapy for 29 months and the total duration of stabilization was 38 months. The remaining patient showed partial response but stopped treatment due to radiological tumor progression during treatment. Although all cases were high risk group for recurrence such as above Milan criteria, vascular invasion and tumor biology, clinical outcomes showed some good results. Therefore, sorafenib may be an acceptable treatment option for recurrent HCC after LT.
MeSH Terms
-
Antineoplastic Agents/*therapeutic use
Carcinoma, Hepatocellular/diagnosis/*drug therapy
Female
Humans
Liver Neoplasms/diagnosis/*drug therapy
*Liver Transplantation
Male
Middle Aged
Neoplasm Recurrence, Local
Niacinamide/*analogs & derivatives/therapeutic use
Phenylurea Compounds/*therapeutic use
Positron-Emission Tomography
Tomography, X-Ray Computed
Antineoplastic Agents
Niacinamide
Phenylurea Compounds
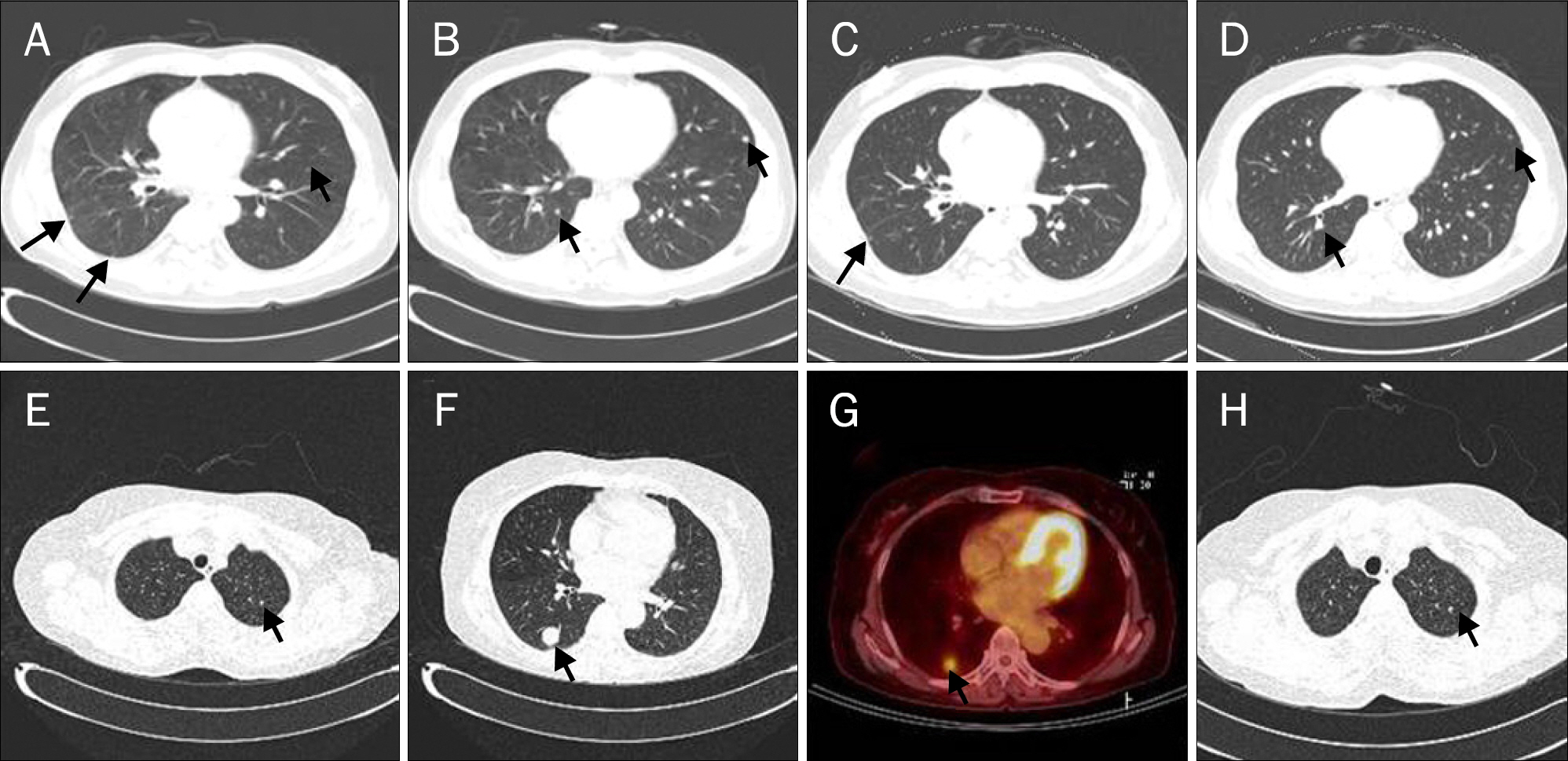
Figure
Reference
-
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.
Article2. Llovet JM, Fuster J, Bruix J. Barcelona-Clínic Liver Cancer Group. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004; 10(2 Suppl 1):S115–S120.
Article3. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009; 10:25–34.
Article4. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003; 362:1907–1917.
Article5. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699.
Article6. Mazzaferro V, Llovet JM, Miceli R, et al. Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.7. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403.
Article8. DuBay D, Sandroussi C, Sandhu L, et al. Liver transplantation for advanced hepatocellular carcinoma using poor tumor differentiation on biopsy as an exclusion criterion. Ann Surg. 2011; 253:166–172.
Article9. Guiteau JJ, Cotton RT, Washburn WK, et al. An early regional experience with expansion of Milan Criteria for liver transplant recipients. Am J Transplant. 2010; 10:2092–2098.
Article10. Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007; 246:502–509. discussion 509–511.11. Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006; 5:835–844.
Article12. Llovet JM, Ricci S, Mazzaferro V, et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008; 359:378–390.
Article13. Bhoori S, Toffanin S, Sposito C, et al. Personalized molecular tar-geted therapy in advanced, recurrent hepatocellular carcinoma after liver transplantation: a proof of principle. J Hepatol. 2010; 52:771–775.
Article14. Lee JO, Kim DY, Lim JH, et al. Palliative chemotherapy for patients with recurrent hepatocellular carcinoma after liver transplantation. J Gastroenterol Hepatol. 2009; 24:800–805.
Article15. Wang Y, Speeg KV, Washburn WK, Halff G. Sirolimus plus sorafenib in treating HCC recurrence after liver transplantation: a case report. World J Gastroenterol. 2010; 16:5518–5522.
Article16. Yoon DH, Ryoo BY, Ryu MH, et al. Sorafenib for recurrent hepatocellular carcinoma after liver transplantation. Jpn J Clin Oncol. 2010; 40:768–773.
Article17. Waidmann O, Hofmann WP, Zeuzem S, Trojan J. mTOR inhibitors and sorafenib for recurrent heptocellular carcinoma after ortho-topic liver transplantation. J Hepatol. 2011; 54:396–398.
Article18. Sposito C, Mariani L, Germini A, et al. Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation: a case-control study. J Hepatol. 2013; 59:59–66.
Article19. Waghray A, Balci B, El-Gazzaz G, et al. Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2013; 27:555–561.
Article20. Alsina AE, Makris A, Nenos V, et al. Can sorafenib increase survival for recurrent hepatocellular carcinoma after liver transplantation? A pilot study. Am Surg. 2014; 80:680–684.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver resection after sorafenib therapy in advanced hepatocellular carcinoma: a case report
- Sorafenib for Recurrent Hepatocellular Carcinoma after Liver Transplantation
- Treatments Other than Sorafenib for Patients with Advanced Hepatocellular Carcinoma
- Successful Sequential Therapy Involving Regorafenib after Failure of Sorafenib in a Patient with Recurrent Hepatocellular Carcinoma after Liver Transplantation
- Sequential regorafenib or nivolumab therapy in recurrent hepatocellular carcinoma with sorafenib failure in liver transplant patients does not improve prognosis