J Pathol Transl Med.
2017 Mar;51(2):152-158. 10.4132/jptm.2016.11.03.
GLUT1 as a Prognostic Factor for Classical Hodgkin's Lymphoma: Correlation with PD-L1 and PD-L2 Expression
- Affiliations
-
- 1Department of Pathology, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Thoracic and Cardiovascular Surgery, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jrhuh@amc.seoul.kr
- KMID: 2372964
- DOI: http://doi.org/10.4132/jptm.2016.11.03
Abstract
- BACKGROUND
Glucose transporter type 1 (GLUT1) expression is linked to glucose metabolism and tissue hypoxia. A recent study reported that GLUT1 was significantly associated with programmed death ligand 1 (PD-L1) as a therapeutic target in relapsed or refractory classical Hodgkin's lymphoma (cHL). The purpose of this study was to measure the expression of GLUT1 and assess its prognostic significance and potential relationships with PD-L1, programmed death ligand 2 (PD-L2), and programmed death-1 (PD-1) expressions in cHL.
METHODS
Diagnostic tissues from 125 patients with cHL treated with doxorubicin, bleomycin, vinblastine, and dacarbazine were evaluated retrospectively via immunohistochemical analysis of GLUT1, PD-L1, PD-L2, and PD-1 expression.
RESULTS
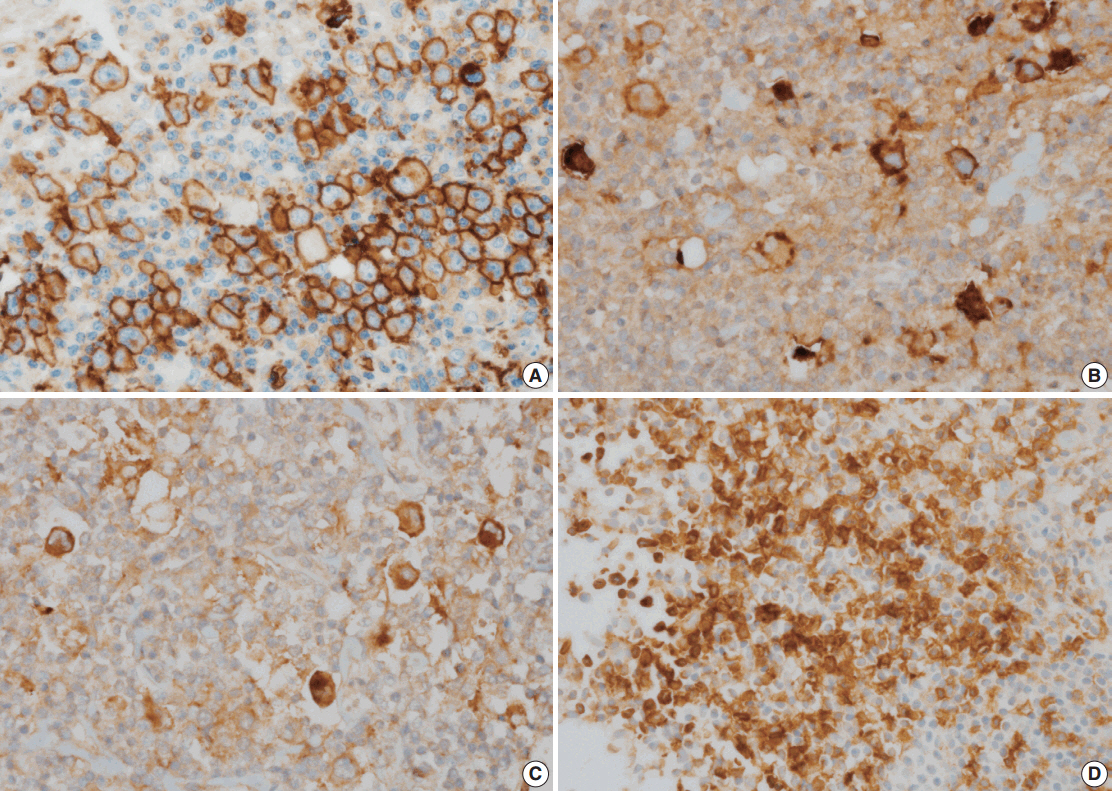
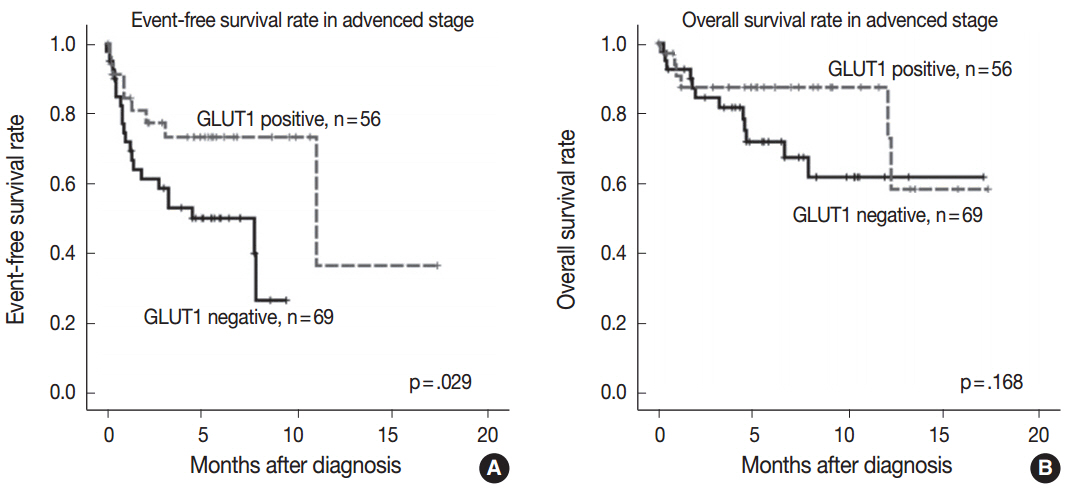
The median follow-up time was 4.83 years (range, 0.08 to 17.33 years). GLUT1, PD-L1, PD-L2, and PD-1 were expressed in 44.8%, 63.2%, 9.6%, and 13.6% of the specimens, respectively. Positive correlations were found between GLUT1 and PD-L1 expression (p = .004) and between GLUT1 and PD-L2 expression (p = .031). GLUT1 expression in Hodgkin/Reed-Sternberg (HRS) cells was not associated with overall survival or event-free survival (EFS) in the entire cohort (p = .299 and p = .143, respectively). A subgroup analysis according to the Ann Arbor stage illustrated that GLUT1 expression in HRS cells was associated with better EFS in advanced-stage disease (p = .029). A multivariate analysis identified GLUT1 as a marginally significant prognostic factor for EFS (p = .068).
CONCLUSIONS
This study suggests that GLUT1 expression is associated with better clinical outcomes in advanced-stage cHL and is significantly associated with PD-L1 and PD-L2 expressions.
Keyword
Figure
Reference
-
1. Björkholm M, Axdorph U, Grimfors G, et al. Fixed versus response-adapted MOPP/ABVD chemotherapy in Hodgkin’s disease: a prospective randomized trial. Ann Oncol. 1995; 6:895–9.2. Quddus F, Armitage JO. Salvage therapy for Hodgkin’s lymphoma. Cancer J. 2009; 15:161–3.
Article3. Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease: international prognostic factors project on advanced Hodgkin’s disease. N Engl J Med. 1998; 339:1506–14.4. Barron CC, Bilan PJ, Tsakiridis T, Tsiani E. Facilitative glucose transporters: implications for cancer detection, prognosis and treatment. Metabolism. 2016; 65:124–39.
Article5. Osugi J, Yamaura T, Muto S, et al. Prognostic impact of the combination of glucose transporter 1 and ATP citrate lyase in node-negative patients with non-small lung cancer. Lung Cancer. 2015; 88:310–8.
Article6. Pinheiro C, Sousa B, Albergaria A, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. 2011; 26:1279–86.7. Haber RS, Rathan A, Weiser KR, et al. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998; 83:34–40.8. Kawamura T, Kusakabe T, Sugino T, et al. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001; 92:634–41.9. Hartmann S, Agostinelli C, Diener J, et al. GLUT1 expression patterns in different Hodgkin lymphoma subtypes and progressively transformed germinal centers. BMC Cancer. 2012; 12:586.
Article10. Shim HK, Lee WW, Park SY, Kim H, Kim SE. Relationship between FDG uptake and expressions of glucose transporter type 1, type 3, and hexokinase-II in Reed-Sternberg cells of Hodgkin lymphoma. Oncol Res. 2009; 17:331–7.
Article11. Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer: preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010; 37:430–9.12. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26:677–704.
Article13. Zeng Z, Shi F, Zhou L, et al. Upregulation of circulating PD-L1/PD-1 is associated with poor post-cryoablation prognosis in patients with HBV-related hepatocellular carcinoma. PLoS One. 2011; 6:e23621.
Article14. Bellmunt J, Mullane SA, Werner L, et al. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015; 26:812–7.
Article15. Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014; 146:15–24.
Article16. Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther. 2014; 7:567–73.
Article17. Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer. 2013; 49:2233–42.
Article18. Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014; 143:512–9.
Article19. Messai Y, Gad S, Noman MZ, et al. Renal cell carcinoma programmed death-ligand 1, a new direct target of hypoxia-inducible factor-2 alpha, is regulated by von Hippel-Lindau gene mutation status. Eur Urol. 2016; 70:623–32.
Article20. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014; 211:781–90.21. Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A mechanism of hypoxia-mediated escape from adaptive immunity in cancer cells. Cancer Res. 2014; 74:665–74.
Article22. Li QQ, Sun YP, Ruan CP, et al. Cellular prion protein promotes glucose uptake through the Fyn-HIF-2alpha-Glut1 pathway to support colorectal cancer cell survival. Cancer Sci. 2011; 102:400–6.23. Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002; 62:4316–24.24. Ruf M, Moch H, Schraml P. PD-L1 expression is regulated by hypoxia inducible factor in clear cell renal cell carcinoma. Int J Cancer. 2016; 139:396–403.
Article25. Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015; 372:311–9.
Article26. Matsumoto T, Jimi S, Migita K, Takamatsu Y, Hara S. Inhibition of glucose transporter 1 induces apoptosis and sensitizes multiple myeloma cells to conventional chemotherapeutic agents. Leuk Res. 2016; 41:103–10.
Article27. Koh YW, Jeon YK, Yoon DH, Suh C, Huh J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol. 2016; 37:7507–14.
Article28. Huh J, Cho K, Heo DS, Kim JE, Kim CW. Detection of Epstein-Barr virus in Korean peripheral T-cell lymphoma. Am J Hematol. 1999; 60:205–14.
Article29. Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010; 17:421–33.
Article30. Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004; 4:253–65.
Article31. Koch A, Lang SA, Wild PJ, et al. Glucose transporter isoform 1 expression enhances metastasis of malignant melanoma cells. Oncotarget. 2015; 6:32748–60.
Article32. Yan S, Wang Y, Chen M, Li G, Fan J. Deregulated SLC2A1 promotes tumor cell proliferation and metastasis in gastric cancer. Int J Mol Sci. 2015; 16:16144–57.
Article33. Uddin S, Hussain AR, Ahmed M, et al. Inhibition of c-MET is a potential therapeutic strategy for treatment of diffuse large B-cell lymphoma. Lab Invest. 2010; 90:1346–56.
Article34. Dinand V, Malik A, Unni R, Arya LS, Pandey RM, Dawar R. Proliferative index and CD15 expression in pediatric classical Hodgkin lymphoma. Pediatr Blood Cancer. 2008; 50:280–3.
Article35. Lee YM, Lee G, Oh TI, et al. Inhibition of glutamine utilization sensitizes lung cancer cells to apigenin-induced apoptosis resulting from metabolic and oxidative stress. Int J Oncol. 2016; 48:399–408.
Article36. Liu Y, Cao Y, Zhang W, et al. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012; 11:1672–82.37. Zhao F, Ming J, Zhou Y, Fan L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother Pharmacol. 2016; 77:963–72.
Article38. Armand P, Shipp MA, Ribrag V, et al. Programmed death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. J Clin Oncol. 2016; Jun. 27. [Epub]. https://doi.org/10.1200/JCO.2016.67.3467.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Programmed Death-Ligand 1 on Immune Cells Is Related to a Better Prognosis in Biliary Tract Cancer
- Overexpression of PD-L1 and PD-L2 Is Associated with Poor Prognosis in Patients with Hepatocellular Carcinoma
- Immunohistochemical expression of programmed death-ligand 1 and CD8 in glioblastomas
- PD-L1 as a Biomarker in Gastric Cancer Immunotherapy
- Correlation of PD-L1 Expression Tested by 22C3 and SP263 in Non-Small Cell Lung Cancer and Its Prognostic Effect on EGFR Mutation–Positive Lung Adenocarcinoma