J Korean Med Sci.
2017 Apr;32(4):642-649. 10.3346/jkms.2017.32.4.642.
Outcome of Reinduction Chemotherapy with a Modified Dose of Idarubicin for Children with Marrow-Relapsed Acute Lymphoblastic Leukemia: Results of the Childhood Acute Lymphoblastic Leukemia (CALL)-0603 Study
- Affiliations
-
- 1Division of Pediatric Hematology/Oncology, Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center Children's Hospital, Seoul, Korea. jjseo@amc.seoul.kr
- 2Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul National University Children's Hospital, Seoul, Korea.
- 3Department of Pediatrics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 4Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Pediatrics, Pusan National University College of Medicine, Busan, Korea.
- 6Department of Pediatrics, Ajou University College of Medicine, Suwon, Korea.
- 7Center for Pediatric Cancer, National Cancer Center, Goyang, Korea.
- KMID: 2371450
- DOI: http://doi.org/10.3346/jkms.2017.32.4.642
Abstract
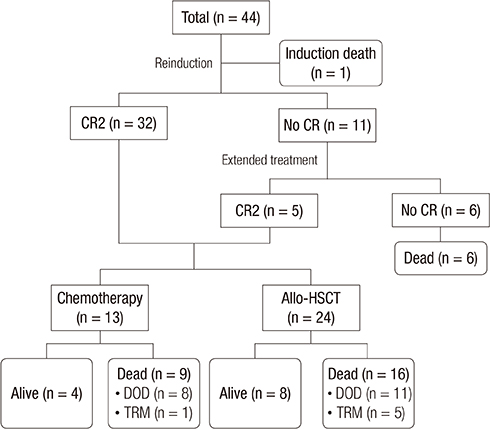
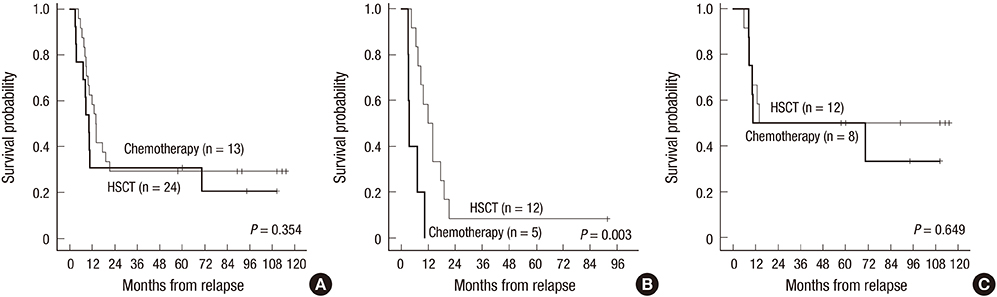
- This multicenter, prospective trial was conducted to develop an effective and safe reinduction regimen for marrow-relapsed pediatric acute lymphoblastic leukemia (ALL) by modifying the dose of idarubicin. Between 2006 and 2009, the trial accrued 44 patients, 1 to 21 years old with first marrow-relapsed ALL. The reinduction regimen comprised prednisolone, vincristine, L-asparaginase, and idarubicin (10 mg/m²/week). The idarubicin dose was adjusted according to the degree of myelosuppression. The second complete remission (CR2) rate was 72.7%, obtained by 54.2% of patients with early relapse < 24 months after initial diagnosis and 95.0% of those with late relapse (P = 0.002). Five patients entered remission with extended treatment, resulting in a final CR2 rate of 84.1%. The CR2 rate was not significantly different according to the idarubicin dose. The induction death rate was 2.3% (1/44). The 5-year event-free and overall survival rates were 22.2% ± 6.4% and 27.3% ± 6.7% for all patients, 4.2% ± 4.1% and 8.3% ± 5.6% for early relapsers, and 43.8% ± 11.4% and 50.0% ± 11.2% for late relapsers, respectively. Early relapse and slow response to reinduction chemotherapy were predictors of poor outcomes. In conclusion, a modified dose of idarubicin was effectively incorporated into the reinduction regimen for late marrow-relapsed ALL with a low toxic death rate. However, the CR2 rate for early relapsers was suboptimal, and the second remission was not durable in most patients.
Keyword
Figure
Cited by 1 articles
-
CD19-Specific CAR-T Cell Treatment of 115 Children and Young Adults with Acute B Lymphoblastic Leukemia: Long-term Follow-up
Yu Wang, Yu-juan Xue, Ying-xi Zuo, Yue-ping Jia, Ai-dong Lu, Hui-min Zeng, Le-ping Zhang
Cancer Res Treat. 2024;56(3):945-955. doi: 10.4143/crt.2023.1205.
Reference
-
1. Pui CH, Yang JJ, Hunger SP, Pieters R, Schrappe M, Biondi A, Vora A, Baruchel A, Silverman LB, Schmiegelow K, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015; 33:2938–2948.2. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015; 373:1541–1552.3. Kim H. Recent advances in the treatment of pediatric acute leukemia. J Korean Med Assoc. 2016; 59:690–697.4. Park KD. How do we prepare ourselves for a new paradigm of medicine to advance the treatment of pediatric acute lymphoblastic leukemia? Blood Res. 2014; 49:3–4.5. Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. 2005; 131:579–587.6. Yang MH, Eun SH, Park CS, Son JA, Kim JY, Ko JW, Ahn DH. A study of the survival rate of childhood cancer in Korea. Cancer Res Treat. 2001; 33:191–198.7. Gaynon PS, Harris RE, Altman AJ, Bostrom BC, Breneman JC, Hawks R, Steele D, Zipf T, Stram DO, Villaluna D, et al. Bone marrow transplantation versus prolonged intensive chemotherapy for children with acute lymphoblastic leukemia and an initial bone marrow relapse within 12 months of the completion of primary therapy: Children’s Oncology Group study CCG-1941. J Clin Oncol. 2006; 24:3150–3156.8. Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2012; 2012:129–136.9. Feig SA, Krailo MD, Harris RE, Baum E, Holcenberg JS, Kaizer H, Steinherz L, Pendergrass TW, Saunders EF, Warkentin PL, et al. Determination of the maximum tolerated dose of idarubicin when used in a combination chemotherapy program of reinduction of childhood ALL at first marrow relapse and a preliminary assessment of toxicity compared to that of daunorubicin: a report from the Childrens Cancer Study Group. Med Pediatr Oncol. 1992; 20:124–129.10. Feig SA, Ames MM, Sather HN, Steinherz L, Reid JM, Trigg M, Pendergrass TW, Warkentin P, Gerber M, Leonard M, et al. Comparison of idarubicin to daunomycin in a randomized multidrug treatment of childhood acute lymphoblastic leukemia at first bone marrow relapse: a report from the Children’s Cancer Group. Med Pediatr Oncol. 1996; 27:505–514.11. Li X, Xu S, Tan Y, Chen J. The effects of idarubicin versus other anthracyclines for induction therapy of patients with newly diagnosed leukaemia. Cochrane Database Syst Rev. 2015; CD010432.12. Yoon JH, Park JA, Kim EK, Kang HJ, Shin HY, Ahn HS. Improvement of induction remission rate by modifying the dose of idarubicin for relapsed childhood acute lymphoblastic leukemia. J Korean Med Sci. 2009; 24:281–288.13. Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, Ebell W, Escherich G, Schrappe M, Klingebiel T, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. 2010; 28:2339–2347.14. Roy A, Cargill A, Love S, Moorman AV, Stoneham S, Lim A, Darbyshire PJ, Lancaster D, Hann I, Eden T, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. 2005; 130:67–75.15. Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: a Children’s Oncology Group Study. [corrected]. J Clin Oncol. 2008; 26:3971–3978.16. Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, Mann G, Hählen K, Göbel U, Klingebiel T, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87. J Clin Oncol. 2005; 23:7942–7950.17. Parker C, Waters R, Leighton C, Hancock J, Sutton R, Moorman AV, Ancliff P, Morgan M, Masurekar A, Goulden N, et al. Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet. 2010; 376:2009–2017.18. Buchanan GR, Rivera GK, Boyett JM, Chauvenet AR, Crist WM, Vietti TJ. Reinduction therapy in 297 children with acute lymphoblastic leukemia in first bone marrow relapse: a Pediatric Oncology Group Study. Blood. 1988; 72:1286–1292.19. Morland BJ, Shaw PJ. Induction toxicity of a modified Memorial Sloan-Kettering-New York II Protocol in children with relapsed acute lymphoblastic leukemia: a single institution study. Med Pediatr Oncol. 1996; 27:139–144.20. Kelly ME, Lu X, Devidas M, Camitta B, Abshire T, Bernstein ML, Billett A, Homans A, Sandler E, Buchanan G. Treatment of relapsed precursor-B acute lymphoblastic leukemia with intensive chemotherapy: POG (Pediatric Oncology Group) study 9411 (SIMAL 9). J Pediatr Hematol Oncol. 2013; 35:509–513.21. Kozlowski P, Åström M, Ahlberg L, Bernell P, Hulegårdh E, Hägglund H, Karlsson K, Markuszewska-Kuczymska A, Tomaszewska-Toporska B, Smedmyr B, et al. High curability via intensive reinduction chemotherapy and stem cell transplantation in young adults with relapsed acute lymphoblastic leukemia in Sweden 2003-2007. Haematologica. 2012; 97:1414–1421.22. Masurekar AN, Parker CA, Shanyinde M, Moorman AV, Hancock JP, Sutton R, Ancliff PJ, Morgan M, Goulden NJ, Fraser C, et al. Outcome of central nervous system relapses in childhood acute lymphoblastic leukaemia--prospective open cohort analyses of the ALLR3 trial. PLoS One. 2014; 9:e108107.23. Benjamin JE, Stein AS. The role of blinatumomab in patients with relapsed/refractory acute lymphoblastic leukemia. Ther Adv Hematol. 2016; 7:142–156.24. Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016; 14:802–808.25. Borgmann A, von Stackelberg A, Hartmann R, Ebell W, Klingebiel T, Peters C, Henze G; Berlin-Frankfurt-Münster Relapse Study Group. Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood. 2003; 101:3835–3839.26. Barrett AJ, Horowitz MM, Pollock BH, Zhang MJ, Bortin MM, Buchanan GR, Camitta BM, Ochs J, Graham-Pole J, Rowlings PA, et al. Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med. 1994; 331:1253–1258.27. Gaynon PS, Qu RP, Chappell RJ, Willoughby ML, Tubergen DG, Steinherz PG, Trigg ME. Survival after relapse in childhood acute lymphoblastic leukemia: impact of site and time to first relapse--the Children’s Cancer Group experience. Cancer. 1998; 82:1387–1395.28. Locatelli F, Moretta F, Rutella S. Management of relapsed acute lymphoblastic leukemia in childhood with conventional and innovative approaches. Curr Opin Oncol. 2013; 25:707–715.29. Eckert C, von Stackelberg A, Seeger K, Groeneveld TW, Peters C, Klingebiel T, Borkhardt A, Schrappe M, Escherich G, Henze G. Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer. 2013; 49:1346–1355.30. Campana D, Coustan-Smith E. Measurements of treatment response in childhood acute leukemia. Korean J Hematol. 2012; 47:245–254.31. Koh KN, Park M, Kim BE, Im HJ, Park CJ, Jang S, Chi HS, Seo JJ. Prognostic significance of minimal residual disease detected by a simplified flow cytometric assay during remission induction chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2010; 53:957–964.32. Jabbour E, Short NJ, Jorgensen JL, Yilmaz M, Ravandi F, Wang SA, Thomas DA, Khoury J, Champlin RE, Khouri I, et al. Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer. 2017; 123:294–302.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improvement of Induction Remission Rate by Modifying the Dose of Idarubicin for Relapsed Childhood Acute Lymphoblastic Leukemia
- A Case of Bone Marrow Necrosis Following Induction Chemotherapy in Childhood Acute Lymphoblastic Leukemia
- Ifosfamide and Etoposide in Relapsed Refractory Childhood Acute Lymphoblastic Leukemia
- A case of bone marrow necrosis in acute lymphoblastic leukemia
- Pancreatitis Induced by 6-mercaptopurine and 6-thioguanine in Childhood Acute Lymphoblastic Leukemia