J Korean Med Sci.
2017 Apr;32(4):605-612. 10.3346/jkms.2017.32.4.605.
Spontaneous Renal Artery Dissection as a Cause of Acute Renal Infarction: Clinical and MDCT Findings
- Affiliations
-
- 1Department of Radiology, Hanyang University College of Medicine, Seoul, Korea. songsy01@gmail.com
- 2Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2371445
- DOI: http://doi.org/10.3346/jkms.2017.32.4.605
Abstract
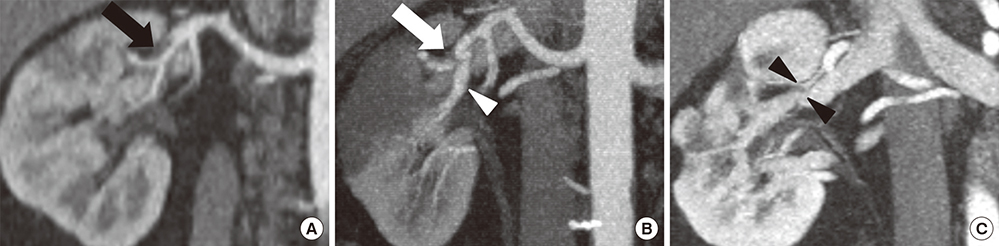
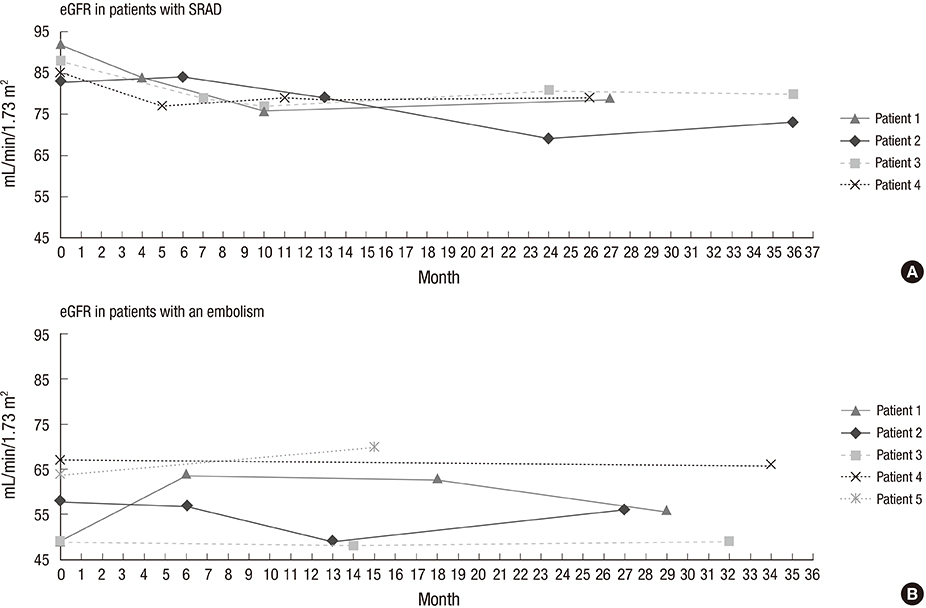
- The purpose of this study was to assess the incidence of spontaneous renal artery dissection (SRAD) as a cause of acute renal infarction, and to evaluate the clinical and multidetector computed tomography (MDCT) findings of SRAD. From November 2011 to January 2014, 35 patients who were diagnosed with acute renal infarction by MDCT were included. We analyzed the 35 MDCT data sets and medical records retrospectively, and compared clinical and imaging features of SRAD with an embolism, using Fisher's exact test and the Mann-Whitney test. The most common cause of acute renal infarction was an embolism, and SRAD was the second most common cause. SRAD patients had new-onset hypertension more frequently than embolic patients. Embolic patients were found to have increased C-reactive protein (CRP) more often than SRAD patients. Laboratory results, including tests for lactate dehydrogenase (LDH) and blood urea nitrogen (BUN), and the BUN/creatinine ratio (BCR) were significantly higher in embolic patients than SRAD patients. Bilateral renal involvement was detected in embolic patients more often than in SRAD patients. MDCT images of SRAD patients showed the stenosis of the true lumen, due to compression by a thrombosed false lumen. None of SRAD patients progressed to an estimated glomerular filtration rate < 60 mL/min/1.73 m2 or to end-stage renal disease during the follow-up period. SRAD is not a rare cause of acute renal infarction, and it has a benign clinical course. It should be considered in a differential diagnosis of acute renal infarction, particularly in patients with new-onset hypertension, unilateral renal involvement, and normal ranges of CRP, LDH, BUN, and BCR.
Keyword
Figure
Reference
-
1. Oh YK, Yang CW, Kim YL, Kang SW, Park CW, Kim YS, Lee EY, Han BG, Lee SH, Kim SH, et al. Clinical characteristics and outcomes of renal infarction. Am J Kidney Dis. 2016; 67:243–250.2. Kanofsky JA, Lepor H. Spontaneous renal artery dissection. Rev Urol. 2007; 9:156–160.3. Mudrick D, Arepally A, Geschwind JF, Ronsivalle JA, Lund GB, Scheel P. Spontaneous renal artery dissection: treatment with coil embolization. J Vasc Interv Radiol. 2003; 14:497–500.4. Pellerin O, Garçon P, Beyssen B, Raynaud A, Rossignol P, Jacquot C, Plouin PF, Sapoval M. Spontaneous renal artery dissection: long-term outcomes after endovascular stent placement. J Vasc Interv Radiol. 2009; 20:1024–1030.5. D’Ambrosio N, Friedman B, Siegel D, Katz D, Newatia A, Hines J. Spontaneous isolated dissection of the celiac artery: CT findings in adults. AJR Am J Roentgenol. 2007; 188:W506-11.6. Jung SC, Lee W, Park EA, Jae HJ, Chung JW, Park JH. Spontaneous dissection of the splanchnic arteries: CT findings, treatment, and outcome. AJR Am J Roentgenol. 2013; 200:219–225.7. Paul JF, Blacher J, Blancher JF, Sapoval M, Safar M, Gaux JC. Spontaneous renal artery dissection revealed by helical CT angiography. Eur Radiol. 2000; 10:783–785.8. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, Neal B, Rodgers A, Ni Mhurchu C, Clark T. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999; 21:1009–1060.9. Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJ. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care. 1995; 18:34–38.10. Richards CL. Urinary tract infections in the frail elderly: issues for diagnosis, treatment and prevention. Int Urol Nephrol. 2004; 36:457–463.11. Li J, Kennedy D, Levine M, Kumar A, Mullen J. Absent hematuria and expensive computerized tomography: case characteristics of emergency urolithiasis. J Urol. 2001; 165:782–784.12. Bolderman R, Oyen R, Verrijcken A, Knockaert D, Vanderschueren S. Idiopathic renal infarction. Am J Med. 2006; 119:356.e9–356.12.13. Korzets Z, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002; 4:781–784.14. Clemente A, Macchi V, Porzionato A, Stecco C, De Caro R, Morra A. CTA and 2D–3D post-processing: radiological signs of fibromuscular dysplasia of renal artery. Surg Radiol Anat. 2009; 31:25–29.15. Huang CC, Lo HC, Huang HH, Kao WF, Yen DH, Wang LM, Huang CI, Lee CH. ED presentations of acute renal infarction. Am J Emerg Med. 2007; 25:164–169.16. Leong FT, Freeman LJ. Acute renal infarction. J R Soc Med. 2005; 98:121–122.17. Lessman RK, Johnson SF, Coburn JW, Kaufman JJ. Renal artery embolism: clinical features and long-term follow-up of 17 cases. Ann Intern Med. 1978; 89:477–482.18. Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, Halimi JM, Karras A, Amoura Z, Jourde-Chiche N, Izzedine H, et al. Acute renal infarction: a case series. Clin J Am Soc Nephrol. 2013; 8:392–398.19. Bumpus HC Jr. A case of renal hypertension. Trans Am Assoc Genitourin Surg. 1945; 37:135–140.20. Lacombe M. Isolated spontaneous dissection of the renal artery. J Vasc Surg. 2001; 33:385–391.21. Bae EJ, Hwang K, Jang HN, Kim MJ, Jeon DH, Kim HJ, Cho HS, Chang SH, Park DJ. A retrospective study of short- and long-term effects on renal function after acute renal infarction. Ren Fail. 2014; 36:1385–1389.22. Prokop M. General principles of MDCT. Eur J Radiol. 2003; 45:Suppl 1. S4–10.23. Grierson C, Uthappa MC, Uberoi R, Warakaulle D. Multidetector CT appearances of splanchnic arterial pathology. Clin Radiol. 2007; 62:717–723.24. Ng C, Gitman M. Isolated spontaneous renal artery dissection. Med Forum. 2009; 11:60–61.25. Renaud S, Leray-Moraguès H, Chenine L, Canaud L, Vernhet-Kovacsik H, Canaud B. Spontaneous renal artery dissection with renal infarction. Clin Kidney J. 2012; 5:261–264.26. Béroniade V, Roy P, Froment D, Pison C. Primary renal artery dissection. Presentation of two cases and brief review of the literature. Am J Nephrol. 1987; 7:382–389.27. Edwards BS, Stanson AW, Holley KE, Sheps SG. Isolated renal artery dissection, presentation, evaluation, management, and pathology. Mayo Clin Proc. 1982; 57:564–571.28. Toto RD, Mitchell HC, Lee HC, Milam C, Pettinger WA. Reversible renal insufficiency due to angiotensin converting enzyme inhibitors in hypertensive nephrosclerosis. Ann Intern Med. 1991; 115:513–519.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spontaneous Renal Artery Dissection in a Patient with Protein C and S Deficiency
- Two Cases of Spontaneous Renal Artery Dissection: Diagnosis using Abdominal Computed Tomography
- A Case of Spontaneous Renal Artery Dissection Causing Renal Infarction in a Previously Healthy Man
- Spontaneous Renal Artery Dissection Complicated by Renal Infarction
- A Case of Spontaneous Renal Artery and Celiac Artery Dissection in Healthy Man