Korean J Physiol Pharmacol.
2017 Jan;21(1):75-82. 10.4196/kjpp.2017.21.1.75.
Acepromazine inhibits hERG potassium ion channels expressed in human embryonic kidney 293 cells
- Affiliations
-
- 1Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. sungkw@catholic.ac.kr
- 2Department of Physiology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 3College of Pharmacy, Integrated Research Institute of Pharmaceutical, The Catholic University of Korea, Seoul 14662, Korea.
- KMID: 2371070
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.75
Abstract
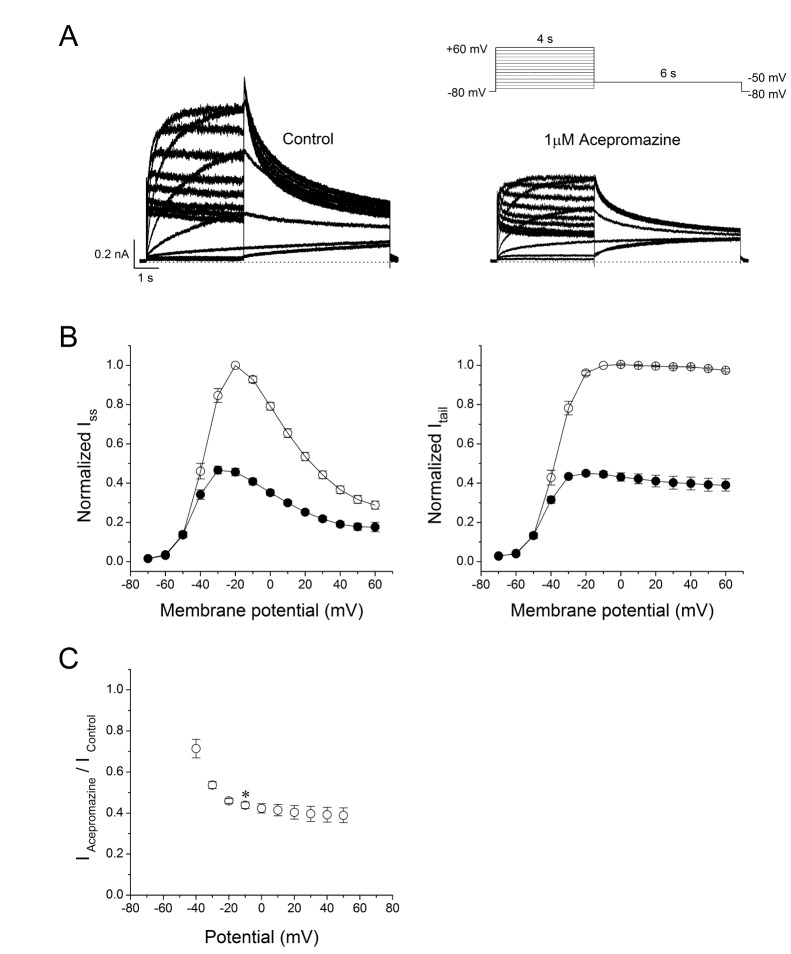
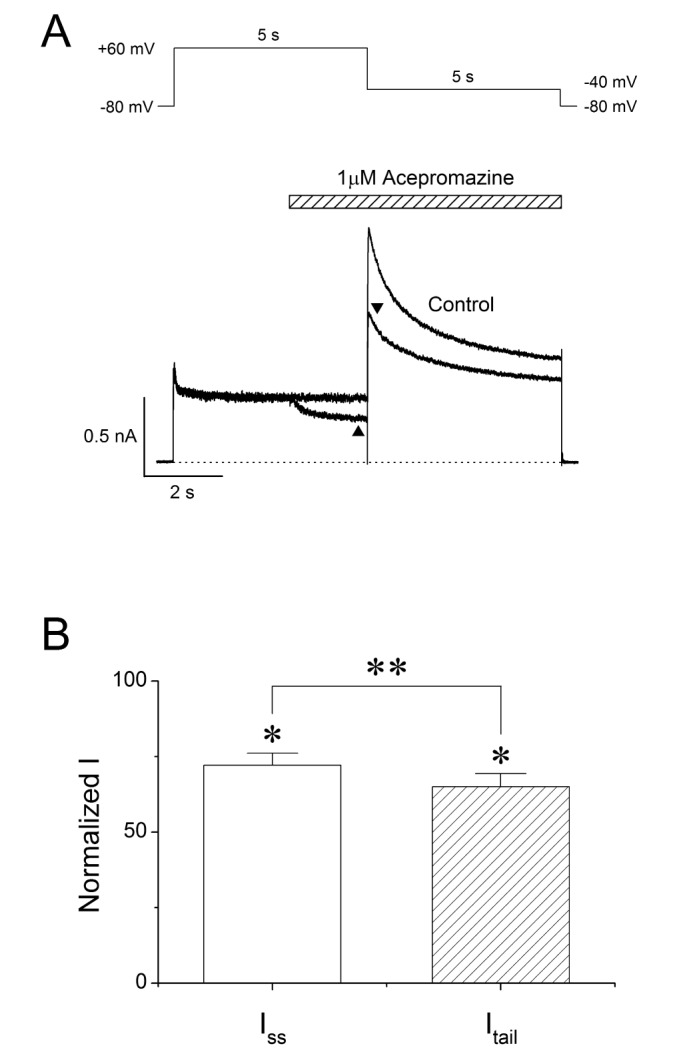
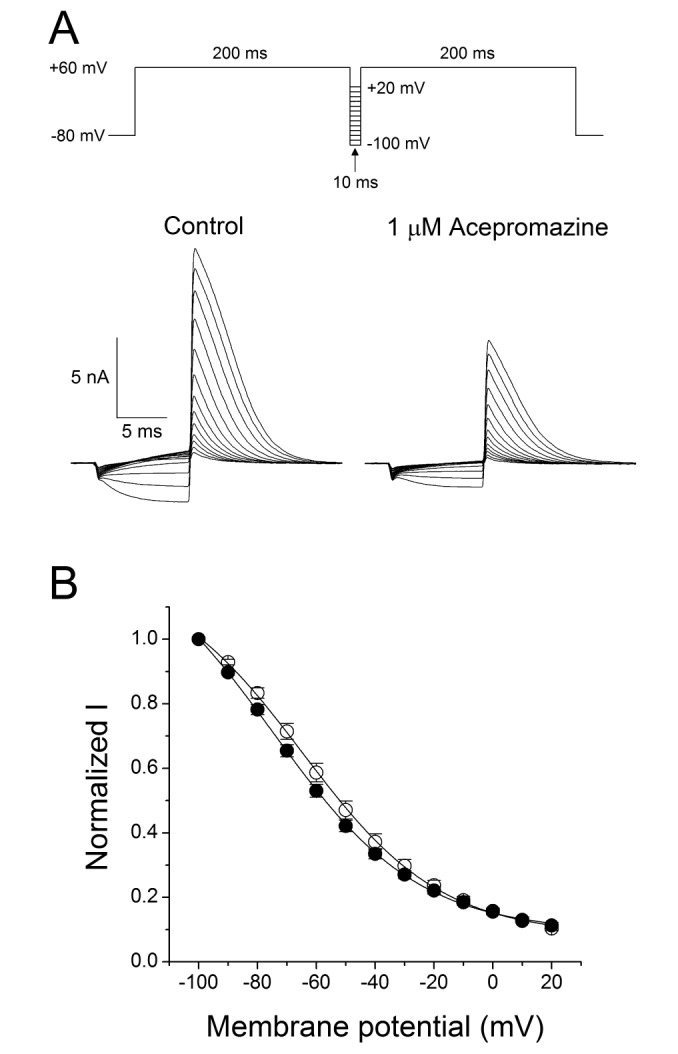
- The effects of acepromazine on human ether-Ã -go-go-related gene (hERG) potassium channels were investigated using whole-cell voltage-clamp technique in human embryonic kidney (HEK293) cells transfected with hERG. The hERG currents were recorded with or without acepromazine, and the steady-state and peak tail currents were analyzed for the evaluating the drug effects. Acepromazine inhibited the hERG currents in a concentration-dependent manner with an ICâ‚…â‚€ value of 1.5 µM and Hill coefficient of 1.1. Acepromazine blocked hERG currents in a voltage-dependent manner between -40 and +10 mV. Before and after application of acepromazine, the half activation potentials of hERG currents changed to hyperpolarizing direction. Acepromazine blocked both the steady-state hERG currents by depolarizing pulse and the peak tail currents by repolarizing pulse; however, the extent of blocking by acepromazine in the repolarizing pulse was more profound than that in the depolarizing pulse, indicating that acepromazine has a high affinity for the open state of the channels, with a relatively lower affinity for the closed state of hERG channels. A fast application of acepromazine during the tail currents inhibited the open state of hERG channels in a concentration-dependent. The steady-state inactivation of hERG currents shifted to the hyperpolarized direction by acepromazine. These results suggest that acepromazine inhibits the hERG channels probably by an open- and inactivated-channel blocking mechanism. Regarding to the fact that the hERG channels are the potential target of drug-induced long QT syndrome, our results suggest that acepromazine can possibly induce a cardiac arrhythmia through the inhibition of hERG channels.
MeSH Terms
Figure
Reference
-
1. Collard JF, Maggs R. Clinical trial of acepromazine maleate in chronic schizophrenia. Br Med J. 1958; 1:1452–1454. PMID: 13536530.
Article2. O'reilly PO, Michaud M, Wojcicki HM, Keogh RP. Acetylpromazine (acepromazine) in the treatment of chronic mental illness. Can Med Assoc J. 1958; 79:381–383. PMID: 13573283.3. Simpson RW. The effects of notensil in chronic mental illness. J Ment Sci. 1958; 104:179–181. PMID: 13514458.
Article4. Urquhart R, Forrest AD. Clinical trial of promazine hydrochloride and acetylpromazine in chronic schizophrenic patients. J Ment Sci. 1959; 105:260–264. PMID: 13641981.
Article5. Rankin DC. Sedatives and tranquilizers. In : Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, Robertson SA, editors. Veterinary anesthesia and analgesia. 5th ed. Oxford: John Wiley & Sons;2015. p. 196–206.6. López-Sanromán FJ, Gómez Cisneros D, Varela del Arco M, Santiago Llorente I, Santos González M. The use of low doses of acepromazine as an aid for lameness diagnosis in horses: An accelerometric evaluation. Vet Comp Orthop Traumatol. 2015; 28:312–317. PMID: 26219640.
Article7. Monteiro ER, Coelho K, Bressan TF, Simões CR, Monteiro BS. Effects of acepromazine-morphine and acepromazine-methadone premedication on the minimum alveolar concentration of isoflurane in dogs. Vet Anaesth Analg. 2016; 43:27–34. PMID: 25880906.
Article8. Perrin KL, Denwood MJ, Grøndahl C, Nissen P, Bertelsen MF. Comparison of etorphine-acepromazine and medetomidine-ketamine anesthesia in captive impala (aepyceros melampus). J Zoo Wildl Med. 2015; 46:870–879. PMID: 26667544.
Article9. Berns SD, Wright JL. Pediatric acepromazine poisoning: the importance of child-resistant packaging for veterinary drugs. Am J Emerg Med. 1993; 11:247–248. PMID: 8489670.
Article10. Algren DA, Ashworth A. Acute acepromazine overdose: clinical effects and toxicokinetic evaluation. J Med Toxicol. 2015; 11:121–123. PMID: 25059809.
Article11. Bryant SM, Mycyk MB. Human exposure to pet prescription medications. Vet Hum Toxicol. 2002; 44:218–219. PMID: 12136968.12. Clutton RE. Attempted suicide with acepromazine maleate: a case report. Vet Hum Toxicol. 1985; 27:391. PMID: 4060560.13. Gaulier JM, Sauvage FL, Pauthier H, Saint-Marcoux F, Marquet P, Lachâtre G. Identification of acepromazine in hair: an illustration of the difficulties encountered in investigating drug-facilitated crimes. J Forensic Sci. 2008; 53:755–759. PMID: 18471229.
Article14. Sterken J, Troubleyn J, Gasthuys F, Maes V, Diltoer M, Verborgh C. Intentional overdose of Large Animal Immobilon. Eur J Emerg Med. 2004; 11:298–301. PMID: 15359207.
Article15. Stowell LI. Suicide with the veterinary drug acepromazine. J Anal Toxicol. 1998; 22:166–168. PMID: 9547414.
Article16. Thomas D, Karle CA, Kiehn J. The cardiac hERG/IKr potassium channel as pharmacological target: structure, function, regulation, and clinical applications. Curr Pharm Des. 2006; 12:2271–2283. PMID: 16787254.
Article17. Raschi E, Vasina V, Poluzzi E, De Ponti F. The hERG K+ channel: target and antitarget strategies in drug development. Pharmacol Res. 2008; 57:181–195. PMID: 18329284.18. Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006; 440:463–469. PMID: 16554806.
Article19. Martinson AS, van Rossum DB, Diatta FH, Layden MJ, Rhodes SA, Martindale MQ, Jegla T. Functional evolution of Erg potassium channel gating reveals an ancient origin for IKr. Proc Natl Acad Sci U S A. 2014; 111:5712–5717. PMID: 24706772.
Article20. Lees-Miller JP, Kondo C, Wang L, Duff HJ. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ Res. 1997; 81:719–726. PMID: 9351446.21. Drolet B, Vincent F, Rail J, Chahine M, Deschênes D, Nadeau S, Khalifa M, Hamelin BA, Turgeon J. Thioridazine lengthens repolarization of cardiac ventricular myocytes by blocking the delayed rectifier potassium current. J Pharmacol Exp Ther. 1999; 288:1261–1268. PMID: 10027867.22. Hoehns JD, Stanford RH, Geraets DR, Skelly KS, Lee HC, Gaul BL. Torsades de pointes associated with chlorpromazine: case report and review of associated ventricular arrhythmias. Pharmacotherapy. 2001; 21:871–883. PMID: 11444585.
Article23. Kim KS, Kim EJ. The phenothiazine drugs inhibit hERG potassium channels. Drug Chem Toxicol. 2005; 28:303–313. PMID: 16051556.
Article24. Thomas D, Wu K, Kathöfer S, Katus HA, Schoels W, Kiehn J, Karle CA. The antipsychotic drug chlorpromazine inhibits HERG potassium channels. Br J Pharmacol. 2003; 139:567–574. PMID: 12788816.
Article25. Chae YJ, Jeon JH, Lee HJ, Kim IB, Choi JS, Sung KW, Hahn SJ. Escitalopram block of hERG potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 2014; 387:23–32. PMID: 24045971.
Article26. Ganapathi SB, Kester M, Elmslie KS. State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy. Am J Physiol Cell Physiol. 2009; 296:C701–C710. PMID: 19244476.27. Dahl SG, Hough E, Hals PA. Phenothiazine drugs and metabolites: molecular conformation and dopaminergic, alpha adrenergic and muscarinic cholinergic receptor binding. Biochem Pharmacol. 1986; 35:1263–1269. PMID: 2870716.
Article28. Hals PA, Hall H, Dahl SG. Phenothiazine drug metabolites: dopamine D2 receptor, alpha 1- and alpha 2-adrenoceptor binding. Eur J Pharmacol. 1986; 125:373–381. PMID: 2874041.29. Peroutka SJ, Synder SH. Relationship of neuroleptic drug effects at brain dopamine, serotonin, alpha-adrenergic, and histamine receptors to clinical potency. Am J Psychiatry. 1980; 137:1518–1522. PMID: 6108081.30. Ashoor A, Lorke D, Nurulain SM, Kury LA, Petroianu G, Yang KH, Oz M. Effects of phenothiazine-class antipsychotics on the function of α7-nicotinic acetylcholine receptors. Eur J Pharmacol. 2011; 673:25–32. PMID: 22044918.
Article31. Hals PA, Hall H, Dahl SG. Muscarinic cholinergic and histamine H1 receptor binding of phenothiazine drug metabolites. Life Sci. 1988; 43:405–412. PMID: 2899826.
Article32. Lee IS, Park TJ, Suh BC, Kim YS, Rhee IJ, Kim KT. Chlorpromazineinduced inhibition of catecholamine secretion by a differential blockade of nicotinic receptors and L-type Ca2+ channels in rat pheochromocytoma cells. Biochem Pharmacol. 1999; 58:1017–1024. PMID: 10509754.33. Schmidt W, Lang K. Life-threatening dysrhythmias in severe thioridazine poisoning treated with physostigmine and transient atrial pacing. Crit Care Med. 1997; 25:1925–1930. PMID: 9366781.
Article34. Wymore RS, Gintant GA, Wymore RT, Dixon JE, McKinnon D, Cohen IS. Tissue and species distribution of mRNA for the IKr-like K+ channel, erg. Circ Res. 1997; 80:261–268. PMID: 9012748.35. Gintant GA, Limberis JT, McDermott JS, Wegner CD, Cox BF. The canine Purkinje fiber: an in vitro model system for acquired long QT syndrome and drug-induced arrhythmogenesis. J Cardiovasc Pharmacol. 2001; 37:607–618. PMID: 11336111.
Article36. Gralinski MR. The dog's role in the preclinical assessment of QT interval prolongation. Toxicol Pathol. 2003; 31(Suppl):11–16. PMID: 12597426.
Article37. Tontodonati M, Fasdelli N, Moscardo E, Giarola A, Dorigatti R. A canine model used to simultaneously assess potential neurobehavioural and cardiovascular effects of candidate drugs. J Pharmacol Toxicol Methods. 2007; 56:265–275. PMID: 17587603.
Article38. Wieder ME, Gray BP, Brown PR, Hudson S, Pearce CM, Paine SW, Hillyer L. Identification of acepromazine and its metabolites in horse plasma and urine by LC-MS/MS and accurate mass measurement. Chromatographia. 2012; 75:635–643.
Article39. Hashem A, Keller H. Disposition, bioavailability and clinical efficacy of orally administered acepromazine in the horse. J Vet Pharmacol Ther. 1993; 16:359–368. PMID: 8230407.
Article40. Daniel WA. Mechanisms of cellular distribution of psychotropic drugs. Significance for drug action and interactions. Prog Neuropsychopharmacol Biol Psychiatry. 2003; 27:65–73. PMID: 12551728.
Article41. Brock N. Acepromazine revisited. Can Vet J. 1994; 35:458–459. PMID: 8076296.42. Darpö B. Spectrum of drugs prolonging QT interval and the incidence of torsades de pointes. Eur Heart J Suppl. 2001; 3:K70–K80.43. Finley MR, Lillich JD, Gilmour RF Jr, Freeman LC. Structural and functional basis for the long QT syndrome: relevance to veterinary patients. J Vet Intern Med. 2003; 17:473–488. PMID: 12892298.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- K+ channel
- Effect of Intracellular ATP on Zn2+ Blockade of KATP Channels in Pancreatic Beta Cells
- Wide Spectrum of Inhibitory Effects of Sertraline on Cardiac Ion Channels
- Physiological Roles and Properties of Ion Channels in Corporal Smooth Muscle
- Effects of Propofol in Voltage-dependent Potassium Channels in Human Neurl Stem Cells