Ann Surg Treat Res.
2017 Feb;92(2):97-104. 10.4174/astr.2017.92.2.97.
Inhibitory effect of sustained perivascular delivery of paclitaxel on neointimal hyperplasia in the jugular vein after open cutdown central venous catheter placement in rats
- Affiliations
-
- 1Department of General Surgery, Wonju Severance Christian Hospital, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 2Department of Surgery, Kangwon National University School of Medicine, Chuncheon, Korea. sukbae75.moon@gmail.com
- 3Department of Surgery, Eulji University Hospital, Daejeon, Korea.
- KMID: 2367922
- DOI: http://doi.org/10.4174/astr.2017.92.2.97
Abstract
- PURPOSE
Inhibitory effect of paclitaxel on neointimal hyperplasia after open cutdown has not been elucidated.
METHODS
For the control group (n = 16), silicone 2.7-Fr catheters were placed via the right external jugular vein with the cutdown method. For the treatment group (n = 16), a mixture of 0.65 mg of paclitaxel and 1 mL of fibrin glue was infiltrated around the exposed vein after cutdown. After scheduled intervals (1, 2, 4, and 8 weeks), the vein segment was harvested and morphometric analysis was performed on cross-sections.
RESULTS
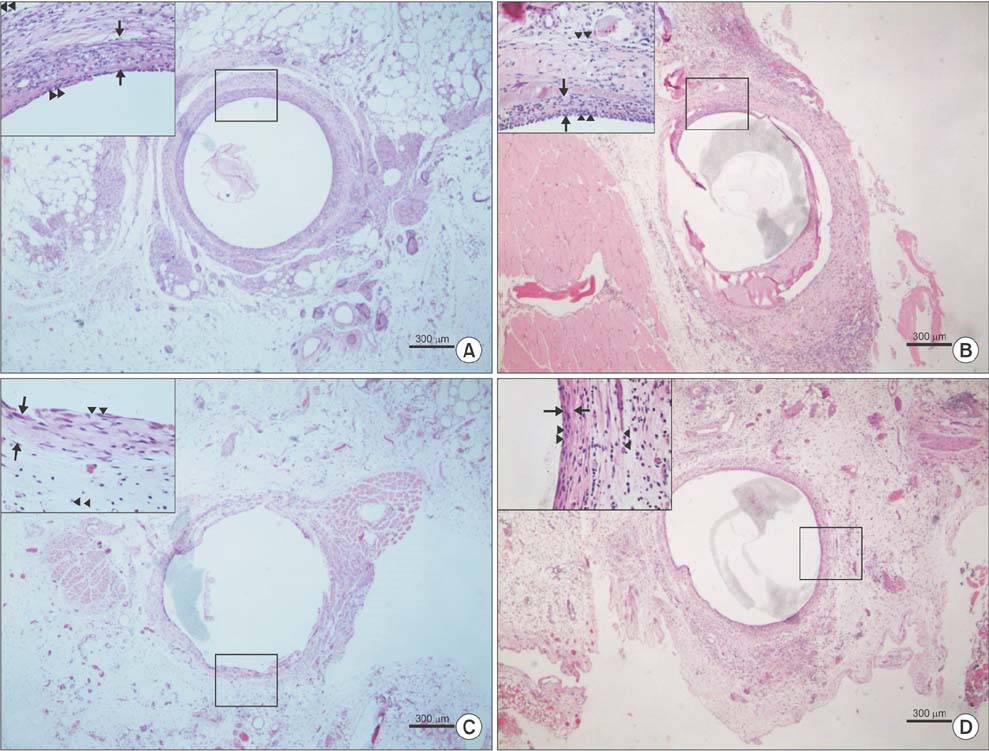
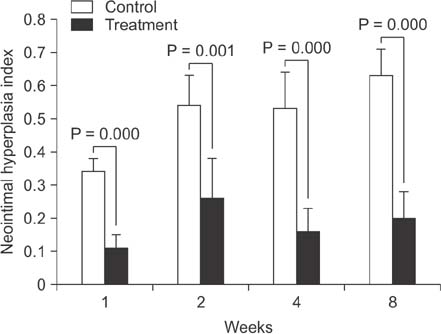
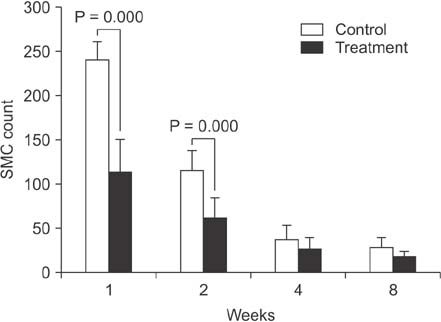
Proliferation of smooth muscle cell (SMC) was strongly suppressed in the treatment group, and the ratio of neointima to vein wall was significantly reduced in the treatment group (8 weeks; 0.63 ± 0.08 vs. 0.2 ± 0.08, P < 0.05). Luminal patency was significantly more preserved in the treatment group, and the luminal area was significantly wider in the paclitaxel-treated group compared to the control group (8 weeks; 1.91 ± 0.43 mm² vs. 5.1 ± 0.43 mm², P < 0.05). Mean SMC counts measured at 1 and 2 weeks after cutdown were significantly lower in the treatment group (2 weeks; 115 ± 22 vs. 62 ± 22). Paclitaxel was undetectable in systemic circulation (<10 ng/mL).
CONCLUSION
Sustained perivascular delivery of paclitaxel with fibrin glue was effective in inhibiting neointimal hyperplasia in rat jugular vein after open cutdown.
Keyword
MeSH Terms
Figure
Reference
-
1. Hong SM, Lee HS, Moon SB. Central venous cutdown in neonates: feasibility as a bedside procedure without general anesthesia. J Pediatr Surg. 2013; 48:1722–1726.2. Kim MJ, Chang HK, Lee MS, Han SJ, Oh JT. Internal jugular vein deformities after central venous catheterisation in neonates: evaluation by Doppler ultrasound. J Paediatr Child Health. 2010; 46:154–158.3. Willetts IE, Ayodeji M, Ramsden WH, Squire R. Venous patency after open central-venous cannulation. Pediatr Surg Int. 2000; 16:411–413.4. Kim S, Kim Y, Moon SB. Histological changes of the unligated vein wall adjacent to the central venous catheter after open cutdown in rats. J Pediatr Surg. 2015; 50:1928–1932.5. Dake MD, Van Alstine WG, Zhou Q, Ragheb AO. Polymer-free paclitaxel-coated Zilver PTX Stents--evaluation of pharmacokinetics and comparative safety in porcine arteries. J Vasc Interv Radiol. 2011; 22:603–610.6. Lee BH, Nam HY, Kwon T, Kim SJ, Kwon GY, Jeon HJ, et al. Paclitaxel-coated expanded polytetrafluoroethylene haemodialysis grafts inhibit neointimal hyperplasia in porcine model of graft stenosis. Nephrol Dial Transplant. 2006; 21:2432–2438.7. Baek I, Hwang J, Park J, Kim H, Park JS, Kim DJ. Paclitaxel coating on the terminal portion of hemodialysis grafts effectively suppresses neointimal hyperplasia in a porcine model. J Vasc Surg. 2015; 61:1575–1582.e1.8. Nakano Y, Ishikawa T, Mutoh M. Long-term angiographic outcomes of sirolimus- and paclitaxel-eluting stent placement in diabetes, long lesions, and small vessels. Cardiovasc Interv Ther. 2015; 30:327–337.9. Laird JR, Hong M. Drug-eluting stents in the superficial femoral artery: The Long and Winding Road. Circulation. 2016; 133:1435–1437.10. De Luca G, Wirianta J, Lee JH, Kaiser C, Di Lorenzo E, Suryapranata H. Sirolimus-eluting versus paclitaxel-eluting stent in primary angioplasty: a pooled patientlevel meta-analysis of randomized trials. J Thromb Thrombolysis. 2014; 38:355–363.11. Hur SH, Cho YK, Nam CW, Kim H, Han SW, Kim YN, et al. Comparison of long-term outcomes following sirolimus-eluting stent vs paclitaxel-eluting stent implantation in patients with long calcified coronary lesions. Clin Cardiol. 2009; 32:633–638.12. Yonemoto H, Ogino S, Nakashima MN, Wada M, Nakashima K. Determination of paclitaxel in human and rat blood samples after administration of low dose paclitaxel by HPLC-UV detection. Biomed Chromatogr. 2007; 21:310–317.13. Kohler TR, Toleikis PM, Gravett DM, Avelar RL. Inhibition of neointimal hyperplasia in a sheep model of dialysis access failure with the bioabsorbable Vascular Wrap paclitaxel-eluting mesh. J Vasc Surg. 2007; 45:1029–1037.14. Kwon JS, Park SS, Kim YG, Son JH, Lee YS, Kim KS, et al. Perivascular delivery of paclitaxel with F-127 pluronic gel inhibits neointimal hyperplasia in a rat carotid artery injury model. Korean Circ J. 2005; 35:221–227.15. Park D, Kim SM, Min SI, Ha J, Kim IG, Min SK. Inhibition of intimal hyperplasia by local perivascular application of rapamycin and imatinib mesilate after carotid balloon injury. J Korean Surg Soc. 2013; 85:296–301.16. Spotnitz WD. Commercial fibrin sealants in surgical care. Am J Surg. 2001; 182:2 Suppl. 8S–14S.17. McEvoy GK. American hospital formulary service drug information. Bethesda (MD): American Society of Health-System Pharmacists;2000.18. Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, et al. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circ Cardiovasc Interv. 2011; 4:495–504.19. Fernandez-Parra R, Laborda A, Lahuerta C, Lostale F, Aramayona J, de Blas I, et al. Pharmacokinetic study of paclitaxel concentration after drug-eluting balloon angioplasty in the iliac artery of healthy and atherosclerotic rabbit models. J Vasc Interv Radiol. 2015; 26:1380–1387.e1.20. Masaki T, Rathi R, Zentner G, Leypoldt JK, Mohammad SF, Burns GL, et al. Inhibition of neointimal hyperplasia in vascular grafts by sustained perivascular delivery of paclitaxel. Kidney Int. 2004; 66:2061–2069.21. Hurwitz CA, Strauss LC, Kepner J, Kretschmar C, Harris MB, Friedman H, et al. Paclitaxel for the treatment of progressive or recurrent childhood brain tumors: a pediatric oncology phase II study. J Pediatr Hematol Oncol. 2001; 23:277–281.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Safe Method of Central Venous Catheterization by Peripheral Venous Cutdown in Infants
- Malposition of central venous catheter in the jugular venous arch via external jugular vein: a case report
- Accidental malpositioning of 9Fr central venous catheter in the right subclavian vein via right internal jugular vein: A case report
- Malposition of a Subclavian Catheter in the Internal Jugular Vein Due to the Direction of a J-type Guidewire End
- Placement of CVP Catheter by EKG