Urogenit Tract Infect.
2016 Dec;11(3):103-108. 10.14777/uti.2016.11.3.103.
Serum CCL11 Levels in Benign Prostatic Hyperplasia and Prostate Cancer
- Affiliations
-
- 1Department of Urology, Hanyang University College of Medicine, Seoul, Korea.
- 2Department of Family Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 3Department of Environmental Biology and Medical Parasitology, Hanyang University College of Medicine, Seoul, Korea. jsryu@hanyang.ac.kr
- 4Department of Internal Medicine, Seoul Seonam Hospital, Seoul, Korea.
- KMID: 2366146
- DOI: http://doi.org/10.14777/uti.2016.11.3.103
Abstract
- PURPOSE
CC-chemokine ligand 11 (CCL11; eotaxin-1), an eosinophil chemoattractant chemokine, has been proposed as a serum marker for prostate cancer (PCa) by two research groups. We investigated the usefulness of CCL11 in diagnosing prostatic diseases, such as benign prostatic hyperplasia (BPH) and PCa.
MATERIALS AND METHODS
CCL11 was measured in the sera of 139 men with BPH, 44 men with PCa, and 45 control men attending an outpatient health-screening clinic. A commercial enzyme-linked immunosorbent assay kit was used to measure CCL11.
RESULTS
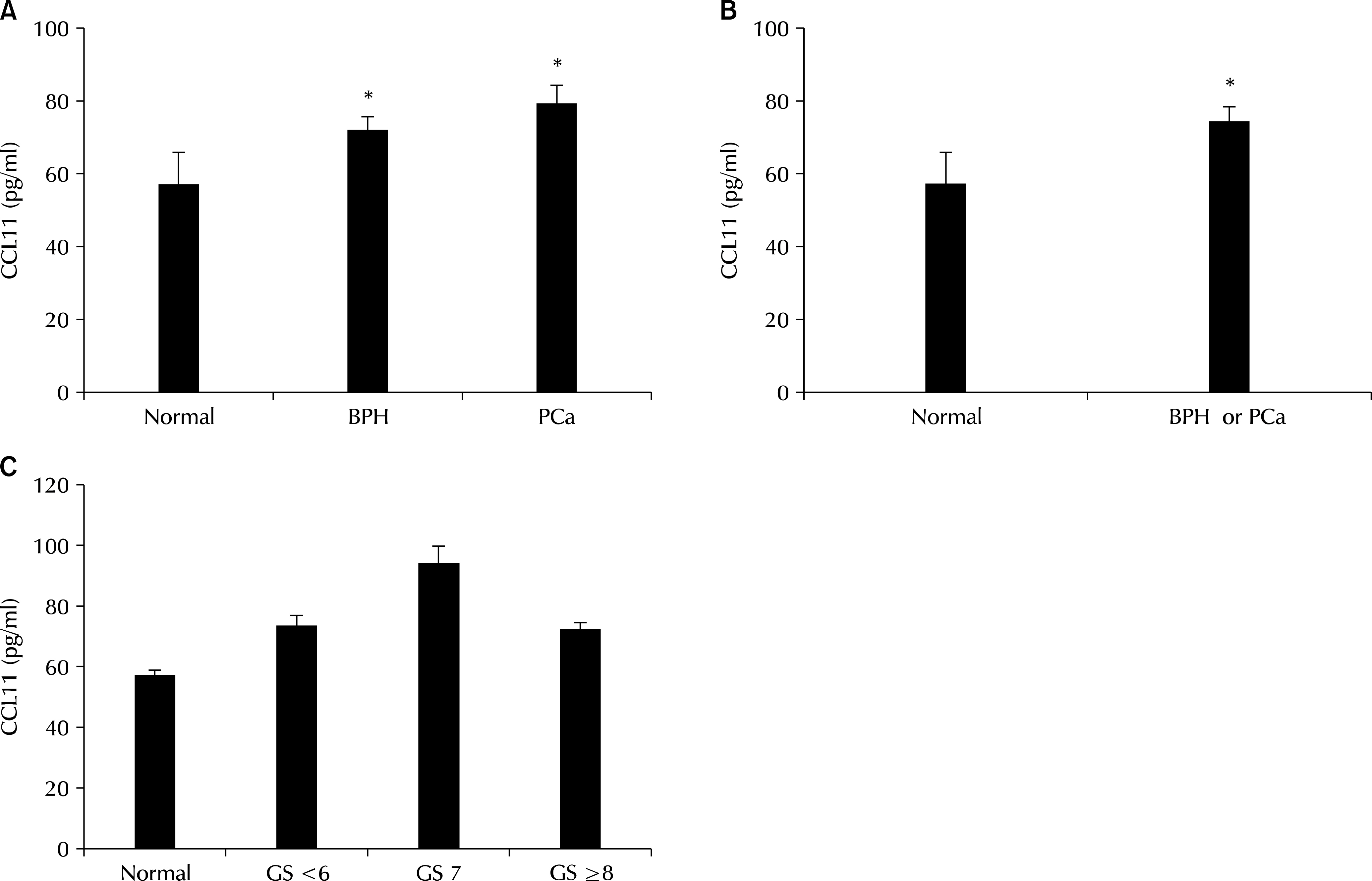
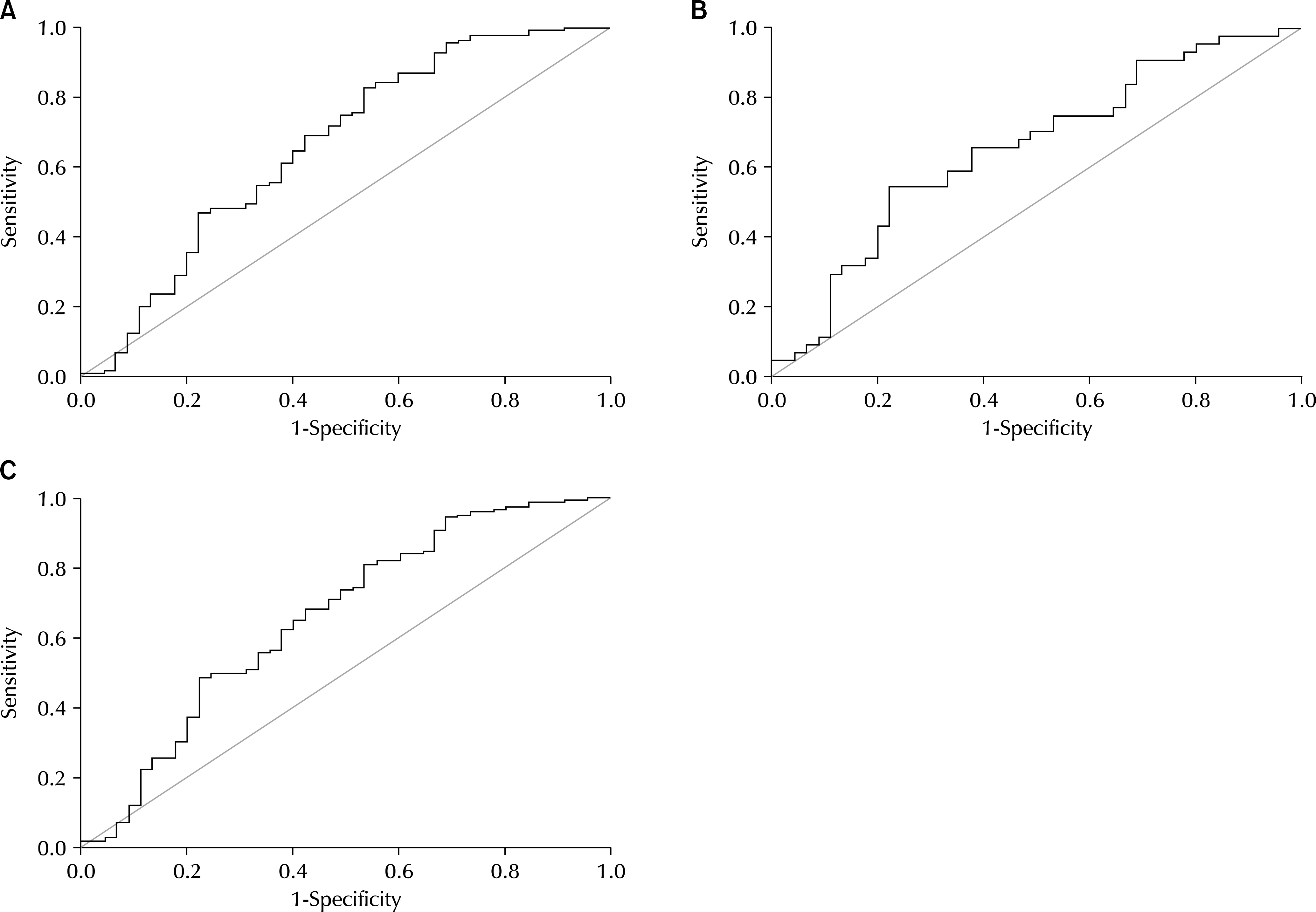
CCL11 concentrations were significantly higher in men with BPH and PCa than in normal men (72.9±3.15 and 80.0±4.91 pg/ml vs. 57.6±8.24). In addition, a receiver operating characteristic (ROC) analysis of serum CCL11 levels showed that the areas under the ROC curves were 0.661 (p=0.001) and 0.654 (p=0.012) for BPH and PCa, respectively, compared with normal men.
CONCLUSIONS
CCL11 may be helpful in diagnosing prostatic diseases, such as BPH and PCa.
MeSH Terms
Figure
Reference
-
1.Matthews AN., Friend DS., Zimmermann N., Sarafi MN., Luster AD., Pearlman E, et al. Eotaxin is required for the baseline level of tissue eosinophils. Proc Natl Acad Sci U S A. 1998. 95:6273–8.
Article2.Solari R., Pease JE., Begg M. Chemokine receptors as therapeutic targets: why aren't there more drugs? Eur J Pharmacol. 2015. 746:363–7.
Article3.Agarwal M., He C., Siddiqui J., Wei JT., Macoska JA. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate. 2013. 73:573–81.
Article4.Heidegger I., Hofer J., Luger M., Pichler R., Klocker H., Horninger W, et al. Is Eotaxin-1 a serum and urinary biomarker for prostate cancer detection and recurrence? Prostate. 2015. 75:1904–9.
Article5.Bianchi-Frias D., Vakar-Lopez F., Coleman IM., Plymate SR., Reed MJ., Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One. 2010. 5:DOI: doi: 10.1371/journal.pone.0012501.
Article6.Wei JT., Calhoun E., Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol. 2005. 173:1256–61.
Article7.Siegel R., Naishadham D., Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012. 62:10–29.
Article8.St Sauver JL., Jacobsen SJ. Inflammatory mechanisms associated with prostatic inflammation and lower urinary tract symptoms. Curr Prostate Rep. 2008. 6:67–73.
Article9.Sfanos KS., De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012. 60:199–215.
Article10.Penna G., Mondaini N., Amuchastegui S., Degli Innocenti S., Carini M., Giubilei G, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007. 51:524–33.
Article11.Bouraoui Y., Ricote M., Garcia-Tunon I., Rodriguez-Berriguete G., Touffehi M., Rais NB, et al. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev. 2008. 32:23–32.
Article12.Macoska JA., Begley LA., Dunn RL., Siddiqui J., Wei JT., Sarma AV. Pilot and feasibility study of serum chemokines as markers to distinguish prostatic disease in men with low total serum PSA. Prostate. 2008. 68:442–52.
Article13.Zhu F., Liu P., Li J., Zhang Y. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol Rep. 2014. 31:2049–54.
Article14.Kelly RS., Vander Heiden MG., Giovannucci E., Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. Cancer Epidemiol Biomarkers Prev. 2016. 25:887–906.
Article15.Collin SM., Martin RM., Metcalfe C., Gunnell D., Albertsen PC., Neal D, et al. Prostate-cancer mortality in the USA and UK in 1975-2004: an ecological study. Lancet Oncol. 2008. 9:445–52.
Article16.Miller DC., Hollenbeck BK. Missing the mark on prostate-specific antigen screening. JAMA. 2011. 306:2719–20.
Article17.Cazares LH., Drake RR., Esquela-Kirscher A., Lance RS., Semmes OJ., Troyer DA. Molecular pathology of prostate cancer. Cancer Biomark. 2010. 9:441–59.
Article18.Martin NE., Mucci LA., Loda M., Depinho RA. Prognostic determinants in prostate cancer. Cancer J. 2011. 17:429–37.
Article19.Netto GJ., Cheng L. Emerging critical role of molecular testing in diagnostic genitourinary pathology. Arch Pathol Lab Med. 2012. 136:372–90.
Article20.Salami SS., Schmidt F., Laxman B., Regan MM., Rickman DS., Scherr D, et al. Combining urinary detection of TMPRSS2: ERG and PCA3 with serum PSA to predict diagnosis of prostate cancer. Urol Oncol. 2013. 31:566–71.21.Gerber BO., Zanni MP., Uguccioni M., Loetscher M., Mackay CR., Pichler WJ, et al. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co-localizing with eosinophils. Curr Biol. 1997. 7:836–43.
Article22.Uguccioni M., Mackay CR., Ochensberger B., Loetscher P., Rhis S., LaRosa GJ, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997. 100:1137–43.
Article23.Sallusto F., Mackay CR., Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997. 277:2005–7.
Article24.Levina V., Nolen BM., Marrangoni AM., Cheng P., Marks JR., Szczepanski MJ, et al. Role of eotaxin-1 signaling in ovarian cancer. Clin Cancer Res. 2009. 15:2647–56.
Article25.Waddell A., Ahrens R., Tsai YT., Sherrill JD., Denson LA., Stein-brecher KA, et al. Intestinal CCL11 and eosinophilic inflammation is regulated by myeloid cell-specific RelA/p65 in mice. J Immunol. 2013. 190:4773–85.
Article26.Nolen BM., Lokshin AE. Targeting CCL11 in the treatment of ovarian cancer. Expert Opin Ther Targets. 2010. 14:157–67.
Article27.Chao PZ., Chou CM., Chen CH. Plasma RANTES and eotaxin levels are correlated with the severity of chronic rhinosinusitis. Eur Arch Otorhinolaryngol. 2012. 269:2343–8.
Article28.Geiger SM., Jardim-Botelho A., Williams W., Alexander N., Diemert DJ., Bethony JM. Serum CCL11 (eotaxin-1) and CCL17 (TARC) are serological indicators of multiple helminth infections and are driven by Schistosoma mansoni infection in humans. Trop Med Int Health. 2013. 18:750–60.29.Byers LA., Holsinger FC., Kies MS., William WN., El-Naggar AK., Lee JJ, et al. Serum signature of hypoxia-regulated factors is associated with progression after induction therapy in head and neck squamous cell cancer. Mol Cancer Ther. 2010. 9:1755–63.
Article30.Johrer K., Zelle-Rieser C., Perathoner A., Moser P., Hager M., Ramoner R, et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005. 11:2459–65.
Article31.Egler RA., Li Y., Dang TA., Peters TL., Leung E., Huang S, et al. An integrated proteomic approach to identifying circulating biomarkers in high-risk neuroblastoma and their potential in relapse monitoring. Proteomics Clin Appl. 2011. 5:532–41.
Article32.Shurin GV., Yurkovetsky ZR., Chatta GS., Tourkova IL., Shurin MR., Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007. 39:123–9.
Article33.Villeda SA., Luo J., Mosher KI., Zou B., Britschgi M., Bieri G, et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011. 477:90–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The role of serum prostate specific antigen in prostatic cancer and benign prostatic hyperplasia
- A Prominently Large Glans penis as a Possible sign of Benign Prostatic Hyperplasia
- The influence of age and endocrine factors on the volume of benign prostatic hyperplasia
- Differences in Expression of bcl-2 and p53 Protein in Prostate Carcinoma and Benigh Prostate Hyperplasia
- Clinical Value of Prostatic Biopsy in Patients with Elevated Serum PSA