Korean Circ J.
2017 Jan;47(1):123-131. 10.4070/kcj.2016.0203.
The Control of Drug Release and Vascular Endothelialization after Hyaluronic Acid-Coated Paclitaxel Multi-Layer Coating Stent Implantation in Porcine Coronary Restenosis Model
- Affiliations
-
- 1The Cardiovascular Convergence Research Center of Chonnam National University Hospital, Designated by Korea Ministry of Health and Welfare, Gwangju, Korea.
- 2Korea Cardiovascular Stent Research Institute, Jangsung, Korea. kim@zuhan.com
- 3Department of Cardiology, Chonnam National University Hospital, Gwangju, Korea.
- 4Division of Cardiology, Department of Internal Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- KMID: 2365291
- DOI: http://doi.org/10.4070/kcj.2016.0203
Abstract
- BACKGROUND AND OBJECTIVES
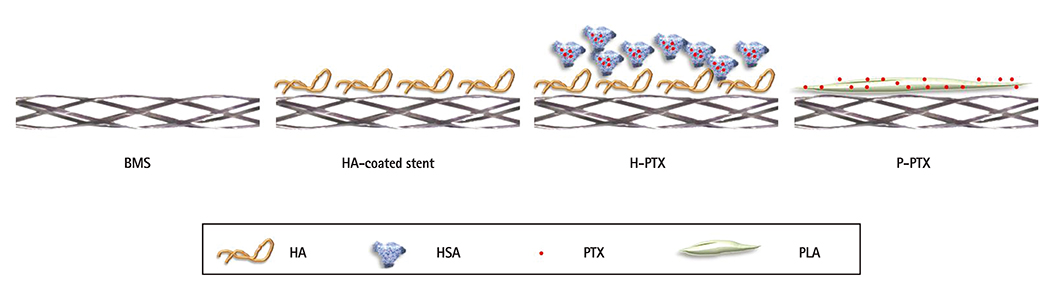
Hyaluronic acid (HA) is highly biocompatible with cells and the extracellular matrix. In contrast to degradation products of a synthetic polymer, degradation products of HA do not acidify the local environment. The aim of this study was to fabricate an HA-coated paclitaxel (PTX)-eluting stent via simple ionic interactions and to evaluate its effects in vitro and in vivo.
MATERIALS AND METHODS
HA and catechol were conjugated by means of an activation agent, and then the stent was immersed in this solution (resulting in a HA-coated stent). After that, PTX was immobilized on the HA-coated stent (resulting in a hyaluronic acid-coated paclitaxel-eluting stent [H-PTX stent]). Study groups were divided into 4 groups: bare metal stent (BMS), HA, H-PTX, and poly (L-lactide)-coated paclitaxel-eluting stent (P-PTX). Stents were randomly implanted in a porcine coronary artery. After 4 weeks, vessels surrounding the stents were isolated and subjected to various analyses.
RESULTS
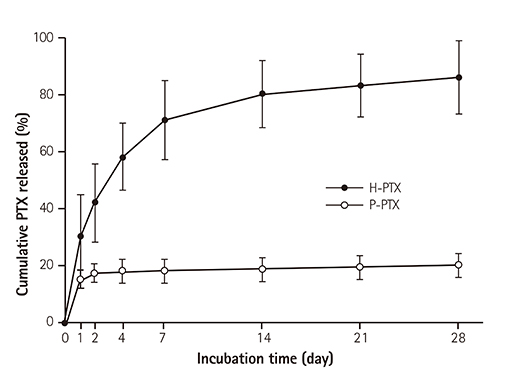
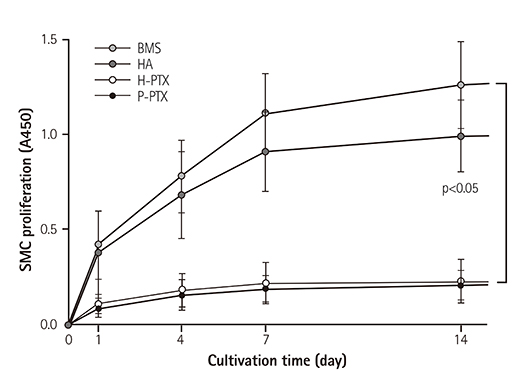
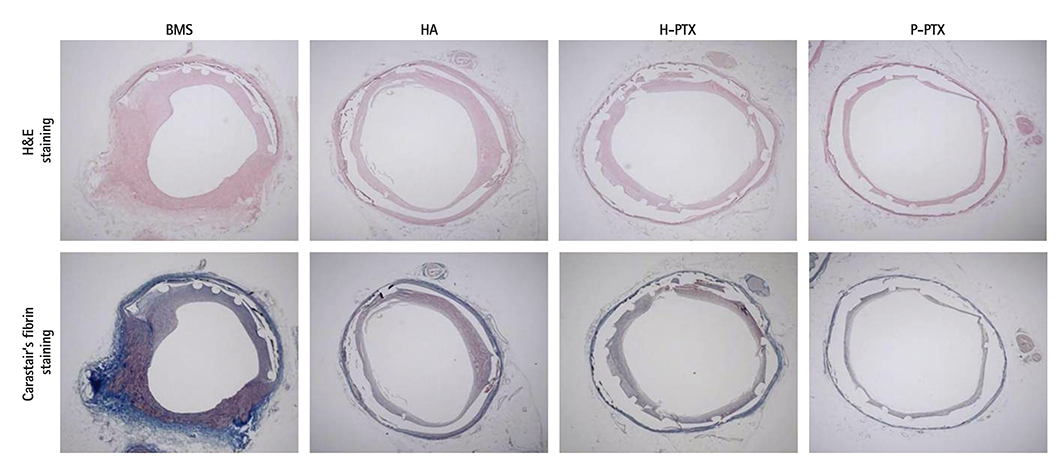
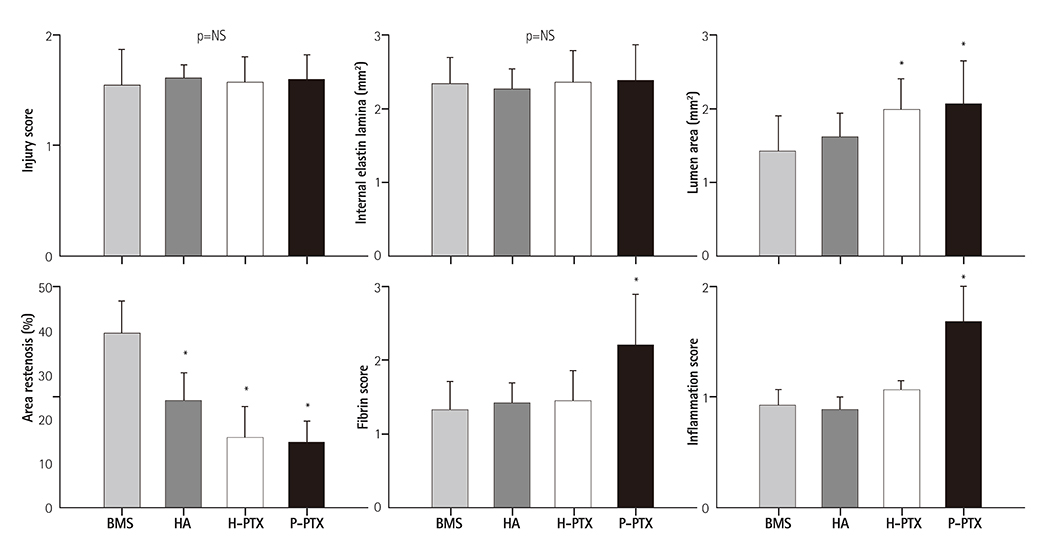
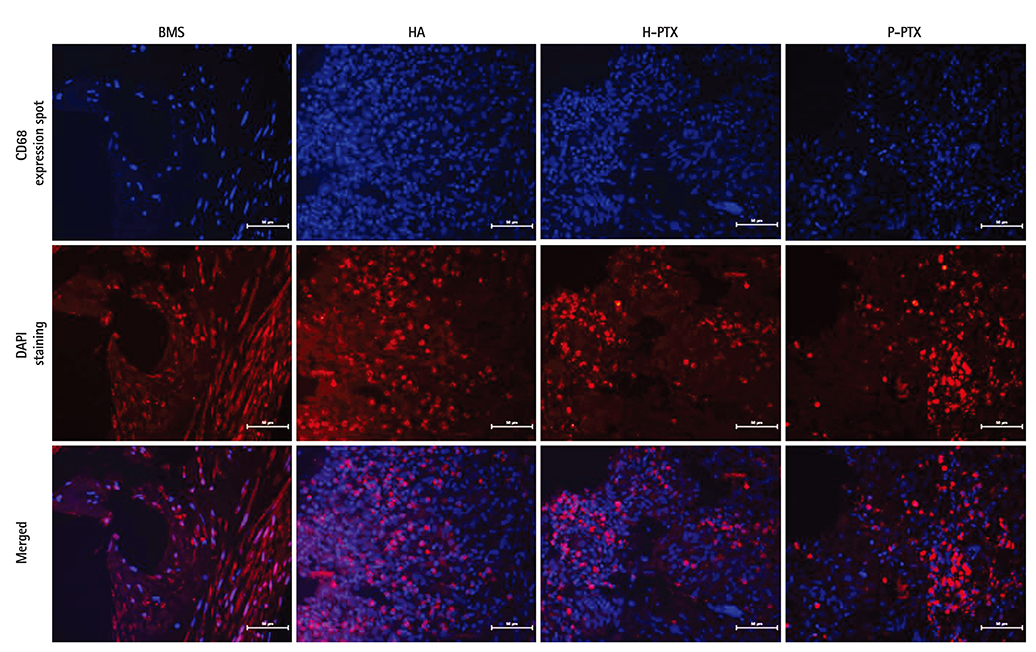
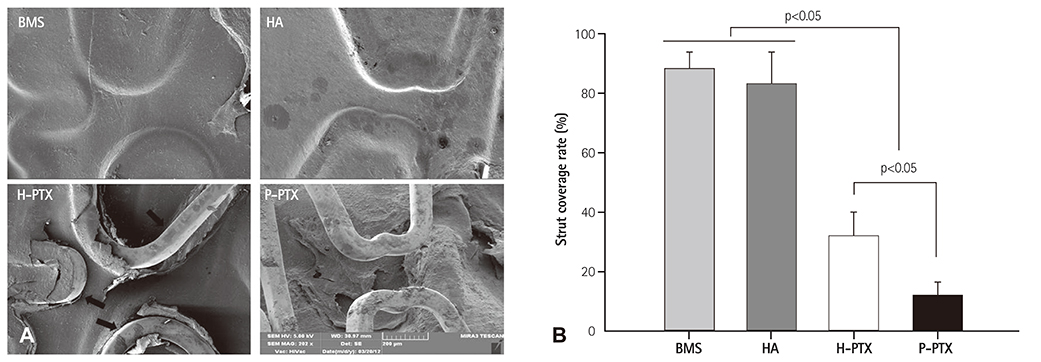
Smoothness of the surface was maintained after expansion of the stent. In contrast to a previous study on a PTX-eluting stent, in this study, the PTX was effectively released up to 14 days (a half amount of PTX in 4 days). The proliferation of smooth muscle cells was successfully inhibited (by 80.5±12.11% at 7 days of culture as compared to the control) by PTX released from the stent. Animal experiments showed that the H-PTX stent does not induce an obvious inflammatory response. Nevertheless, restenosis was clearly decreased in the H-PTX stent group (9.8±3.25%) compared to the bare-metal stent group (29.7±8.11%).
CONCLUSION
A stent was stably coated with PTX via simple ionic interactions with HA. Restenosis was decreased in the H-PTX group. These results suggest that HA, a natural polymer, is suitable for fabrication of drug-eluting stents (without inflammation) as an alternative to a synthetic polymer.
MeSH Terms
Figure
Reference
-
1. Kirtane AJ, Gupta A, Iyengar S, et al. Safety and efficacy of drug-eluting and bare metal stents: comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009; 119:3198–3206.2. Kamath KR, Barry JJ, Miller KM. The Taxus drug-eluting stent: a new paradigm in controlled drug delivery. Adv Drug Deliv Rev. 2006; 58:412–436.3. Kee WJ, Jeong MH, Jang SY, et al. Very late stent thrombosis due to neointimal rupture after paclitaxel-eluting stent implantation. Korean Circ J. 2011; 41:754–758.4. Herdeg C, Oberhoff M, Baumbach A, et al. Local paclitaxel delivery for the prevention of restenosis: biological effects and efficacy in vivo. J Am Coll Cardiol. 2000; 35:1969–1976.5. Albrecht T, Speck U, Baier C, Wolf KJ, Böhm M, Scheller B. Reduction of stenosis due to intimal hyperplasia after stent supported angioplasty of peripheral arteries by local administration of paclitaxel in swine. Invest Radiol. 2007; 42:579–585.6. Regar E, Sianos G, Serruys PW. Stent development and local drug delivery. Br Med Bull. 2001; 59:227–248.7. McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004; 364:1519–1521.8. Stone GW, Moses JW, Ellis SG, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007; 356:998–1008.9. Nebeker JR, Virmani R, Bennett CL, et al. Hypersensitivity cases associated with drug-eluting coronary stents: a review of available cases from the Research on Adverse Drug Events and Reports (RADAR) project. J Am Coll Cardiol. 2006; 47:175–181.10. Otsuka Y, Chronos NA, Apkarian RP, Robinson KA. Scanning electron microscopic analysis of defects in polymer coatings of three commercially available stents: comparison of BiodivYsio, Taxus and Cypher stents. J Invasive Cardiol. 2007; 19:71–76.11. Nakazawa G, Vorpahl M, Finn AV, Narula J, Virmani R. One step forward and two steps back with drug-eluting-stents: from preventing restenosis to causing late thrombosis and nouveau atherosclerosis. JACC Cardiovasc Imaging. 2009; 2:625–628.12. Gouëffic Y, Guilluy C, Guérin P, Patra P, Pacaud P, Loirand G. Hyaluronan induces vascular smooth muscle cell migration through RHAMM-mediated PI3K-dependent Rac activation. Cardiovasc Res. 2006; 72:339–348.13. Barbucci R, Lamponi S, Magnani A, et al. Influence of Sulfation on Platelet Aggregation and Activation with Differentially Sulfated Hyaluronic Acids. J Thromb Thrombolysis. 1998; 6:109–115.14. Buszman P, Trznadel S, Zurakowski A, et al. Prospective registry evaluating safety and efficacy of cobalt-chromium stent implantation in patients with de novo coronary lesions. Kardiol Pol. 2007; 65:1041–1046. discussion 1047-8.15. Bae IH, Lim KS, Park JK, et al. Mechanical behavior and in vivo properties of newly designed bare metal stent for enhanced flexibility. J Indst Eng Chem. 2015; 21:1295–1300.16. Ding D, Tang X, Cao X, et al. Novel self-assembly endows human serum albumin nanoparticles with an enhanced antitumor efficacy. AAPS PharmSciTech. 2014; 15:213–222.17. Che HL, Bae IH, Lim KS, et al. Novel fabrication of microRNA nanoparticle-coated coronary stent for prevention of post-angioplasty restenosis. Korean Circ J. 2016; 46:23–32.18. Qian Z, Haessler M, Lemos JA, et al. Targeting vascular injury using Hantavirus-pseudotyped lentiviral vectors. Mol Ther. 2006; 13:694–704.19. Schwartz RS, Edelman E, Virmani R, et al. Drug-eluting stents in preclinical studies: updated consensus recommendations for preclinical evaluation. Circ Cardiovasc Interv. 2008; 1:143–153.20. Kolodgie FD, John M, Khurana C, et al. Sustained reduction of in-stent neointimal growth with the use of a novel systemic nanoparticle paclitaxel. Circulation. 2002; 106:1195–1198.21. Rodrigues SN, Gonçalves IC, Martins MC, Barbosa MA, Ratner BD. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials. 2006; 27:5357–5367.22. Jabara R, Chronos N, Conway D, Molema W, Robinson K. Evaluation of a novel slow-release paclitaxel-eluting stent with a bioabsorbable polymeric surface coating. JACC Cardiovasc Interv. 2008; 1:81–87.23. Seidel CL. Cellular heterogeneity of the vascular tunica media. Implications for vessel wall repair. Arterioscler Thromb Vasc Biol. 1997; 17:1868–1871.24. Waugh J, Wagstaff AJ. The paclitaxel (TAXUS)-eluting stent: a review of its use in the management of de novo coronary artery lesions. Am J Cardiovasc Drugs. 2004; 4:257–268.25. Popma JJ, Califf RM, Topol EJ. Clinical trials of restenosis after coronary angioplasty. Circulation. 1991; 84:1426–1436.26. Clowes AW, Clowes MM. Kinetics of cellular proliferation after arterial injury. II. Inhibition of smooth muscle growth by heparin. Lab Invest. 1985; 52:611–616.27. Baek S, March KL. Gene therapy for restenosis: getting nearer the heart of the matter. Circ Res. 1998; 82:295–305.28. Park S, Bhang SH, La WG, Seo J, Kim BS, Char K. Dual roles of hyaluronic acids in multilayer films capturing nanocarriers for drug-eluting coatings. Biomaterials. 2012; 33:5468–5477.29. Ceonzo K, Gaynor A, Shaffer L, Kojima K, Vacanti CA, Stahl GL. Polyglycolic acid-induced inflammation: role of hydrolysis and resulting complement activation. Tissue Eng. 2006; 12:301–308.30. Commandeur S, van Beusekom HM, van der Giessen WJ. Polymers, drug release, and drug-eluting stents. J Interv Cardiol. 2006; 19:500–506.31. Bangalore S, Toklu B, Amoroso N, et al. Bare metal stents, durable polymer drug eluting stents, and biodegradable polymer drug eluting stents for coronary artery disease: mixed treatment comparison meta-analysis. BMJ. 2013; 347:f6625.32. Meng S, Liu Z, Shen L, et al. The effect of a layer-by-layer chitosan-heparin coating on the endothelialization and coagulation properties of a coronary stent system. Biomaterials. 2009; 30:2276–2283.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- A Study of Drug Content and Cell Cytotoxicity of Paclitaxel-eluting Stents Coated with Various Biopolymer
- The preventive effects of the heparin-coated coronary stent in a porcine coronary stent restenosis model
- The Effect of the Probucol-Loaded BiodivYsioTM DD Stent on Inhibition of Neointimal Proliferation in Porcine Coronary Stent Restenosis Model
- No-Reflow Phenomenon During Treatment of Coronary In-Stent Restenosis With a Paclitaxel-Coated Balloon Catheter