J Clin Neurol.
2016 Jan;12(1):85-92. 10.3988/jcn.2016.12.1.85.
Epilepsy and Other Neuropsychiatric Manifestations in Children and Adolescents with 22q11.2 Deletion Syndrome
- Affiliations
-
- 1Department of Pediatrics, CHA Gangnam Medical Center, CHA University, Seoul, Korea.
- 2Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea. tsko@amc.seoul.kr
- 3Department of Medical Genetics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Psychiatry, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2364924
- DOI: http://doi.org/10.3988/jcn.2016.12.1.85
Abstract
- BACKGROUND AND PURPOSE
22q11.2 deletion syndrome (22q11.2DS) is the most common microdeletion syndrome. Epilepsy and other neuropsychiatric (NP) manifestations of this genetic syndrome are not uncommon, but they are also not well-understood. We sought to identify the characteristics of epilepsy and other associated NP manifestations in patients with 22q11.2DS.
METHODS
We retrospectively analyzed the medical records of 145 child and adolescent patients (72 males and 73 females) with genetically diagnosed 22q11.2DS. The clinical data included seizures, growth chart, psychological reports, development characteristics, school performance, other clinical manifestations, and laboratory findings.
RESULTS
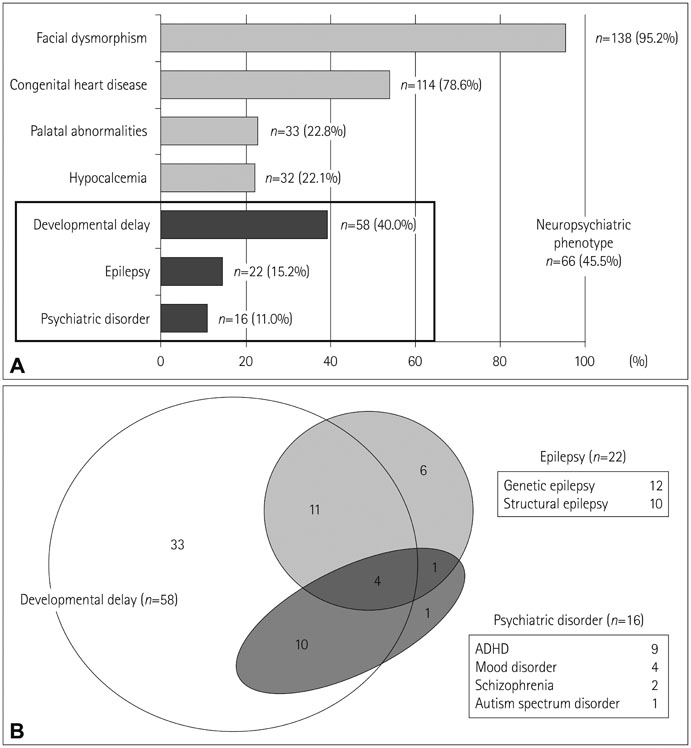
Of the 145 patients with 22q11.2DS, 22 (15.2%) had epileptic seizures, 15 (10.3%) had developmental delay, and 5 (3.4%) had a psychiatric illness. Twelve patients with epilepsy were classified as genetic epilepsy whereas the remaining were classified as structural, including three with malformations of cortical development. Patients with epilepsy were more likely to display developmental delay (odds ratio=3.98; 95% confidence interval=1.5-10.5; p=0.005), and developmental delay was more common in patients with structural epilepsy than in those with genetic epilepsy.
CONCLUSIONS
Patients with 22q11.2DS have a high risk of epilepsy, which in these cases is closely related to other NP manifestations. This implies that this specific genetic locus is critically linked to neurodevelopment and epileptogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Shaikh TH, Kurahashi H, Saitta SC, O'Hare AM, Hu P, Roe BA, et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000; 9:489–501.
Article2. Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007; 370:1443–1452.
Article3. Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010; 11:402–416.
Article4. Schneider M, Debbané M, Bassett AS, Chow EW, Fung WL, van den Bree M, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014; 171:627–639.
Article5. Michaelovsky E, Gothelf D, Korostishevsky M, Frisch A, Burg M, Carmel M, et al. Association between a common haplotype in the COMT gene region and psychiatric disorders in individuals with 22q11.2DS. Int J Neuropsychopharmacol. 2008; 11:351–363.
Article6. Eliez S, Blasey CM. Chromosome 22q11 deletion and brain structure. Br J Psychiatry. 2001; 179:270.
Article7. Schaer M, Glaser B, Ottet MC, Schneider M, Bach Cuadra M, Debbané M, et al. Regional cortical volumes and congenital heart disease: a MRI study in 22q11.2 deletion syndrome. J Neurodev Disord. 2010; 2:224–234.
Article8. Jonas RK, Montojo CA, Bearden CE. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry. 2014; 75:351–360.
Article9. Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry. 2013; 18:1153–1165.
Article10. Gur RE, Yi JJ, McDonald-McGinn DM, Tang SX, Calkins ME, Whinna D, et al. Neurocognitive development in 22q11.2 deletion syndrome: comparison with youth having developmental delay and medical comorbidities. Mol Psychiatry. 2014; 19:1205–1211.
Article11. Kao A, Mariani J, McDonald-McGinn DM, Maisenbacher MK, Brooks-Kayal AR, Zackai EH, et al. Increased prevalence of unprovoked seizures in patients with a 22q11.2 deletion. Am J Med Genet A. 2004; 129A:29–34.
Article12. Lemke JR, Beck-Wödl S, Zankl A, Riegel M, Krämer G, Dorn T, et al. Juvenile myoclonic epilepsy with photosensitivity in a female with Velocardiofacial syndrome (del(22)(q11.2))--causal relationship or coincidence? Seizure. 2009; 18:660–663.
Article13. Robin NH, Taylor CJ, McDonald-McGinn DM, Zackai EH, Bingham P, Collins KJ, et al. Polymicrogyria and deletion 22q11.2 syndrome: window to the etiology of a common cortical malformation. Am J Med Genet A. 2006; 140:2416–2425.
Article14. Castro A, Rodrigues N, Pereira M, Gonçalves C. Bilateral polymicrogyria: always think in chromosome 22q11.2 deletion syndromes. BMJ Case Rep. 2011; 2011.
Article15. Sztriha L, Guerrini R, Harding B, Stewart F, Chelloug N, Johansen JG. Clinical, MRI, and pathological features of polymicrogyria in chromosome 22q11 deletion syndrome. Am J Med Genet A. 2004; 127A:313–317.
Article16. Schaer M, Schmitt JE, Glaser B, Lazeyras F, Delavelle J, Eliez S. Abnormal patterns of cortical gyrification in velo-cardio-facial syndrome (deletion 22q11.2): an MRI study. Psychiatry Res. 2006; 146:1–11.
Article17. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010; 51:676–685.
Article18. Carvill GL, Mefford HC. Microdeletion syndromes. Curr Opin Genet Dev. 2013; 23:232–239.
Article19. McDonald-McGinn DM, Sullivan KE. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Medicine (Baltimore). 2011; 90:1–18.
Article20. Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 2009; 48:1060–1068.
Article21. Ryan AK, Goodship JA, Wilson DI, Philip N, Levy A, Seidel H, et al. Spectrum of clinical features associated with interstitial chromosome 22q11 deletions: a European collaborative study. J Med Genet. 1997; 34:798–804.
Article22. Piccione M, Vecchio D, Cavani S, Malacarne M, Pierluigi M, Corsello G. The first case of myoclonic epilepsy in a child with a de novo 22q11.2 microduplication. Am J Med Genet A. 2011; 155A:3054–3059.
Article23. Striano P, Coppola A, Paravidino R, Malacarne M, Gimelli S, Robbiano A, et al. Clinical significance of rare copy number variations in epilepsy: a case-control survey using microarray-based comparative genomic hybridization. Arch Neurol. 2012; 69:322–330.
Article24. Mullen SA, Carvill GL, Bellows S, Bayly MA, Trucks H, Lal D, et al. Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology. 2013; 81:1507–1514.
Article25. Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. Neuroimage. 2009; 46:373–381.
Article26. Ronan L, Alhusaini S, Scanlon C, Doherty CP, Delanty N, Fitzsimons M. Widespread cortical morphologic changes in juvenile myoclonic epilepsy: evidence from structural MRI. Epilepsia. 2012; 53:651–658.
Article27. Campbell LE, Daly E, Toal F, Stevens A, Azuma R, Catani M, et al. Brain and behaviour in children with 22q11.2 deletion syndrome: a volumetric and voxel-based morphometry MRI study. Brain. 2006; 129(Pt 5):1218–1228.
Article28. Hultman CS, Riski JE, Cohen SR, Burstein FD, Boydston WR, Hudgins RJ, et al. Chiari malformation, cervical spine anomalies, and neurologic deficits in velocardiofacial syndrome. Plast Reconstr Surg. 2000; 106:16–24.
Article29. Yi JJ, Tang SX, McDonald-McGinn DM, Calkins ME, Whinna DA, Souders MC, et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2014; 165B:137–147.
Article30. Fountain DM, Schaer M, Mutlu AK, Schneider M, Debbané M, Eliez S. Congenital heart disease is associated with reduced cortical and hippocampal volume in patients with 22q11.2 deletion syndrome. Cortex. 2014; 57:128–142.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Large Congenital Epigastric Hernia with DiGeorge Syndrome: A Case Report
- A Case of Bernard-Soulier Syndrome Associated with 22q11.2 Deletion Syndrome
- Erratum to: Epilepsy and Other Neuropsychiatric Manifestations in Children and Adolescents with 22q11.2 Deletion Syndrome
- Important Consideration in Choosing Antipsychotics in the Treatment of Patients with 22q11.2 Deletion Syndrome: Risk of Convulsion
- Bilateral Striopallidodentate Calcinosis in Chromosome 22q11.2 Deletion Syndrome