Korean J Ophthalmol.
2015 Apr;29(2):131-137. 10.3341/kjo.2015.29.2.131.
Efficacy of the Mineral Oil and Hyaluronic Acid Mixture Eye Drops in Murine Dry Eye
- Affiliations
-
- 1Department of Ophthalmology and Research Institute of Medical Sciences, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea. kcyoon@jnu.ac.kr
- 2Kim's Eye Clinic of the 21st Century, Seoul, Korea.
- 3Department of Anatomy, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2363720
- DOI: http://doi.org/10.3341/kjo.2015.29.2.131
Abstract
- PURPOSE
To investigate the therapeutic effects of mineral oil (MO) and hyaluronic acid (HA) mixture eye drops on the tear film and ocular surface in a mouse model of experimental dry eye (EDE).
METHODS
Eye drops consisting of 0.1% HA alone or mixed with 0.1%, 0.5%, or 5.0% MO were applied to desiccating stress-induced murine dry eyes. Tear volume, corneal irregularity score, tear film break-up time (TBUT), and corneal fluorescein staining scores were measured at 5 and 10 days after treatment. Ten days after treatment, goblet cells in the conjunctiva were counted after Periodic acid-Schiff staining.
RESULTS
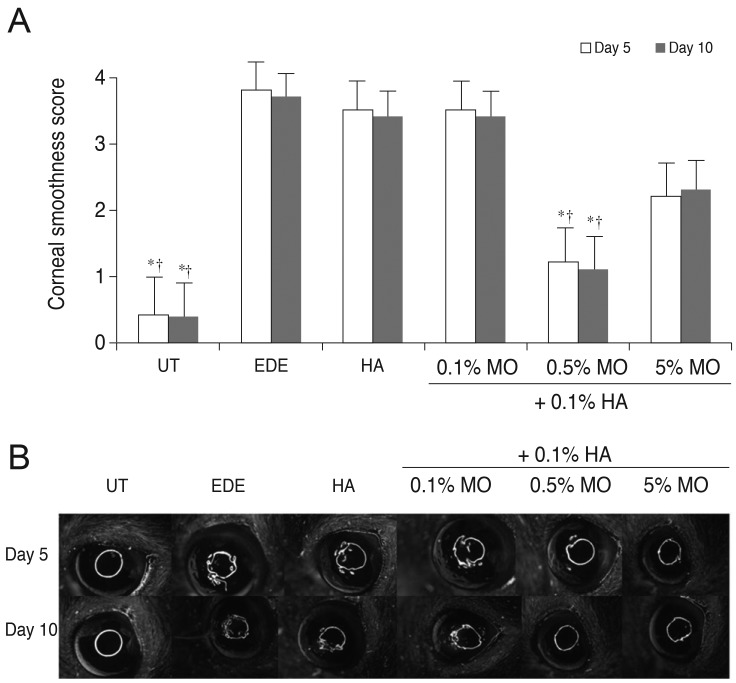
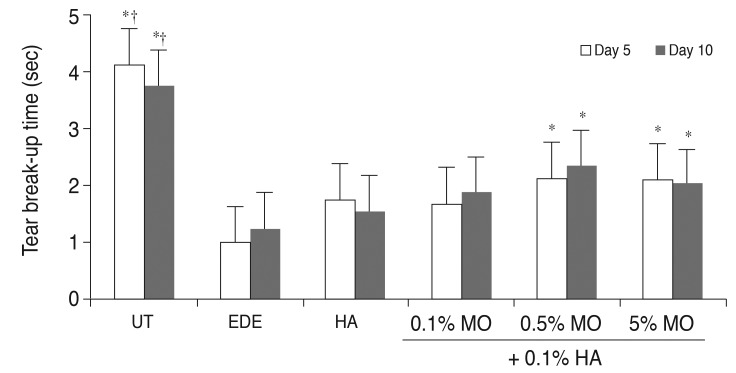
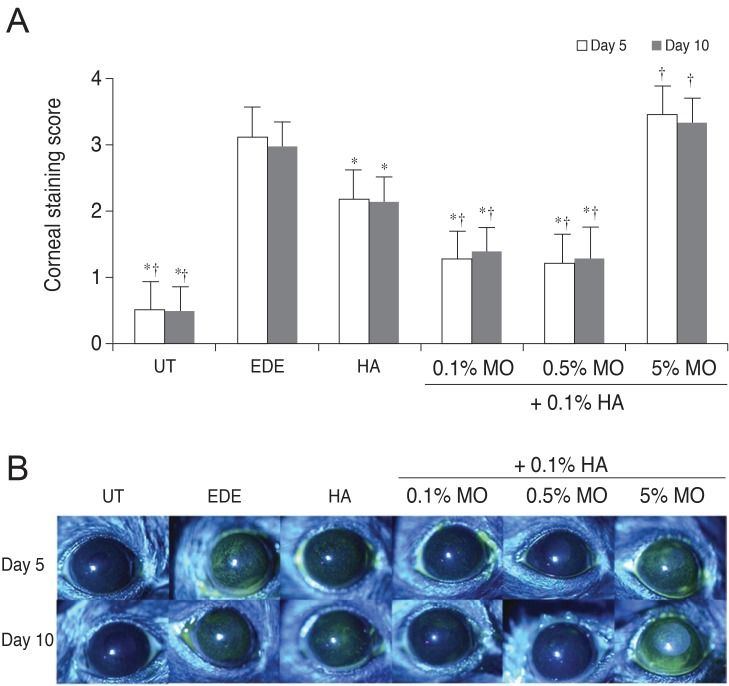
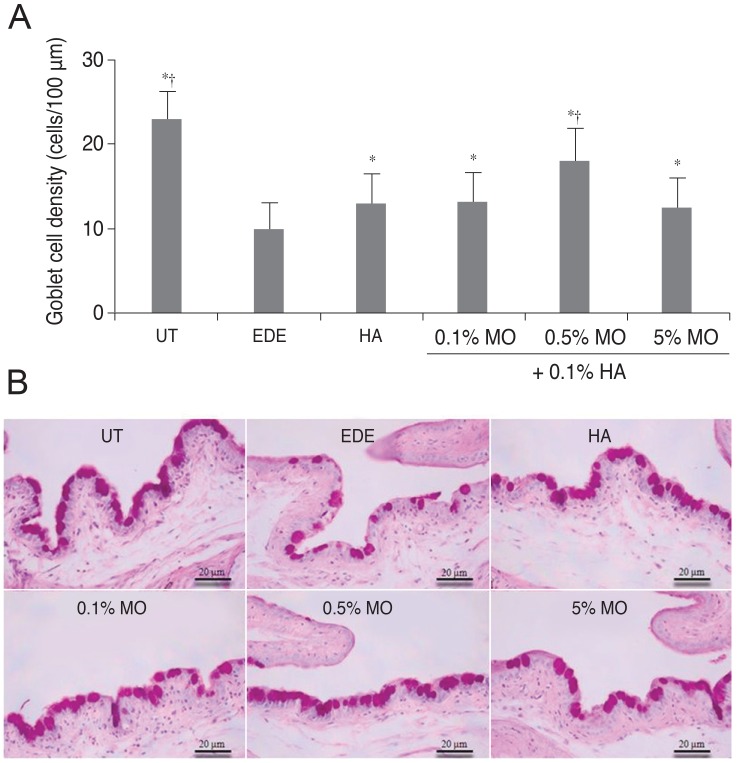
There was no significant difference in the tear volume between desiccating stress-induced groups. The corneal irregularity score was lower in the 0.5% MO group compared with the EDE and HA groups. The 0.5% and 5.0% MO groups showed a significant improvement in TBUT compared with the EDE group. Mice treated with 0.1% and 0.5% MO mixture eye drops showed a significant improvement in fluorescein staining scores compared with the EDE group and the HA group. The conjunctival goblet cell count was higher in the 0.5% MO group compared with the EDE group and HA group.
CONCLUSIONS
The MO and HA mixture eye drops had a beneficial effect on the tear films and ocular surface of murine dry eye. The application of 0.5% MO and 0.1% HA mixture eye drops could improve corneal irregularity, the corneal fluorescein staining score, and conjunctival goblet cell count compared with 0.1% HA eye drops in the treatment of EDE.
Keyword
MeSH Terms
-
Animals
Conjunctiva/*drug effects/pathology
Cornea/metabolism
Disease Models, Animal
Drug Combinations
Dry Eye Syndromes/*drug therapy/metabolism
Emollients/administration & dosage
Female
Goblet Cells/drug effects/metabolism/pathology
Hyaluronic Acid/*administration & dosage
Mice
Mice, Inbred C57BL
Mineral Oil/*administration & dosage
Ophthalmic Solutions
Tears/*metabolism
Viscosupplements/administration & dosage
Drug Combinations
Emollients
Hyaluronic Acid
Mineral Oil
Ophthalmic Solutions
Viscosupplements
Figure
Cited by 1 articles
-
Comparison of Eye Protection Methods of Ointment Instillation under General Anesthesia
Seung Hoon Yoo, Hyuna A Kim, Sang Il Ahn, Soon Im Kim, Jin Kwon Chung
J Korean Ophthalmol Soc. 2015;56(7):1012-1019. doi: 10.3341/jkos.2015.56.7.1012.
Reference
-
1. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007; 5:75–92. PMID: 17508116.2. Cuevas M, Gonzalez-Garcia MJ, Castellanos E, et al. Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by Meibomian gland dysfunction (MGD). Curr Eye Res. 2012; 37:855–863. PMID: 22632103.
Article3. Gupta H, Jain S, Mathur R, et al. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007; 14:507–515. PMID: 18027180.
Article4. Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006; 25:900–907. PMID: 17102664.5. McCann LC, Tomlinson A, Pearce EI, Papa V. Effectiveness of artificial tears in the management of evaporative dry eye. Cornea. 2012; 31:1–5. PMID: 21968605.
Article6. McDonald CC, Kaye SB, Figueiredo FC, et al. A randomised, crossover, multicentre study to compare the performance of 0.1% (w/v) sodium hyaluronate with 1.4% (w/v) polyvinyl alcohol in the alleviation of symptoms associated with dry eye syndrome. Eye (Lond). 2002; 16:601–607. PMID: 12194076.
Article7. Wysenbeek YS, Loya N, Ben Sira I, et al. The effect of sodium hyaluronate on the corneal epithelium. An ultrastructural study. Invest Ophthalmol Vis Sci. 1988; 29:194–199. PMID: 3338879.8. Sand BB, Marner K, Norn MS. Sodium hyaluronate in the treatment of keratoconjunctivitis sicca A double masked clinical trial. Acta Ophthalmol (Copenh). 1989; 67:181–183. PMID: 2658462.9. DeLuise VP, Peterson WS. The use of topical Healon tears in the management of refractory dry-eye syndrome. Ann Ophthalmol. 1984; 16:823–824. PMID: 6508097.10. Stuart JC, Linn JG. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann Ophthalmol. 1985; 17:190–192. PMID: 3873200.11. Rawlings AV, Lombard KJ. A review on the extensive skin benefits of mineral oil. Int J Cosmet Sci. 2012; 34:511–518. PMID: 22994201.
Article12. Wang IJ, Lin IC, Hou YC, Hu FR. A comparison of the effect of carbomer-, cellulose- and mineral oil-based artificial tear formulations. Eur J Ophthalmol. 2007; 17:151–159. PMID: 17415686.
Article13. Yoon KC, De Paiva CS, Qi H, et al. Desiccating environmental stress exacerbates autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008; 30:212–221. PMID: 17988834.
Article14. Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren’s Syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006; 176:3950–3957. PMID: 16547229.15. De Paiva CS, Corrales RM, Villarreal AL, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006; 83:526–535. PMID: 16643899.
Article16. De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007; 48:2553–2560. PMID: 17525184.17. Villareal AL, Farley W, Pflugfelder SC. Effect of topical ophthalmic epinastine and olopatadine on tear volume in mice. Eye Contact Lens. 2006; 32:272–276. PMID: 17099387.
Article18. Li Z, Choi W, Oh HJ, Yoon KC. Effectiveness of topical infliximab in a mouse model of experimental dry eye. Cornea. 2012; 31(Suppl 1):S25–S31. PMID: 23038030.
Article19. Li Z, Woo JM, Chung SW, et al. Therapeutic effect of topical adiponectin in a mouse model of desiccating stress-induced dry eye. Invest Ophthalmol Vis Sci. 2013; 54:155–162. PMID: 23211823.
Article20. De Paiva CS, Corrales RM, Villarreal AL, et al. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis Sci. 2006; 47:2847–2856. PMID: 16799024.
Article21. Dogru M, Erturk H, Shimazaki J, et al. Tear function and ocular surface changes with topical mitomycin (MMC) treatment for primary corneal intraepithelial neoplasia. Cornea. 2003; 22:627–639. PMID: 14508259.
Article22. Xiao X, He H, Lin Z, et al. Therapeutic effects of epidermal growth factor on benzalkonium chloride-induced dry eye in a mouse model. Invest Ophthalmol Vis Sci. 2012; 53:191–197. PMID: 22159022.
Article23. Vogel R, Crockett RS, Oden N, et al. Demonstration of efficacy in the treatment of dry eye disease with 0.18% sodium hyaluronate ophthalmic solution (vismed, rejena). Am J Ophthalmol. 2010; 149:594–601. PMID: 20346777.
Article24. Brignole F, Pisella PJ, Dupas B, et al. Efficacy and safety of 0.18% sodium hyaluronate in patients with moderate dry eye syndrome and superficial keratitis. Graefes Arch Clin Exp Ophthalmol. 2005; 243:531–538. PMID: 15965673.
Article25. Nakamura M, Hikida M, Nakano T. Concentration and molecular weight dependency of rabbit corneal epithelial wound healing on hyaluronan. Curr Eye Res. 1992; 11:981–986. PMID: 1451529.
Article26. Stern ME, Beuerman RW, Fox RI, et al. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998; 17:584–589. PMID: 9820935.27. Adamia S, Maxwell CA, Pilarski LM. Hyaluronan and hyaluronan synthases: potential therapeutic targets in cancer. Curr Drug Targets Cardiovasc Haematol Disord. 2005; 5:3–14. PMID: 15720220.
Article28. Turino GM, Cantor JO. Hyaluronan in respiratory injury and repair. Am J Respir Crit Care Med. 2003; 167:1169–1175. PMID: 12714341.
Article29. Toole BP. Hyaluronan promotes the malignant phenotype. Glycobiology. 2002; 12:37R–42R.
Article30. Lerner LE, Schwartz DM, Hwang DG, et al. Hyaluronan and CD44 in the human cornea and limbal conjunctiva. Exp Eye Res. 1998; 67:481–484. PMID: 9820796.
Article31. Sullivan LJ, McCurrach F, Lee S, et al. Efficacy and safety of 0.3% carbomer gel compared to placebo in patients with moderate-to-severe dry eye syndrome. Ophthalmology. 1997; 104:1402–1408. PMID: 9307633.
Article32. Wang TJ, Wang IJ, Ho JD, et al. Comparison of the clinical effects of carbomer-based lipid-containing gel and hydroxypropyl-guar gel artificial tear formulations in patients with dry eye syndrome: a 4-week, prospective, open-label, randomized, parallel-group, noninferiority study. Clin Ther. 2010; 32:44–52. PMID: 20171410.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined Effect of Rebamipide 2% and Hyaluronic Acid 0.15% on Dry Eye after Cataract Surgery
- Characteristics of Hyaluronic Acid and Its Use in Ocular Surface Diseases Including Dry Eye
- Recent treatment of dry eye
- Efficacy of Topical Cyclosporine in Mild Dry Eye Patients Having Refractive Surgery
- Assessment of the Compliance with 0.1% Cyclosporine A in Dry-Eye Patients with Sjögren's Syndrome