J Vet Sci.
2016 Mar;17(1):103-109. 10.4142/jvs.2016.17.1.103.
Time-dependent low-field MRI characteristics of canine blood: an in vitro study
- Affiliations
-
- 1College of Veterinary Medicine, Chonbuk National University Specialized Campus, Iksan 54596, Korea. kclee@chonbuk.ac.kr
- 2College of Health Sciences, Radiologic Science, Cheongju University, Cheongju 28503, Korea.
- KMID: 2363341
- DOI: http://doi.org/10.4142/jvs.2016.17.1.103
Abstract
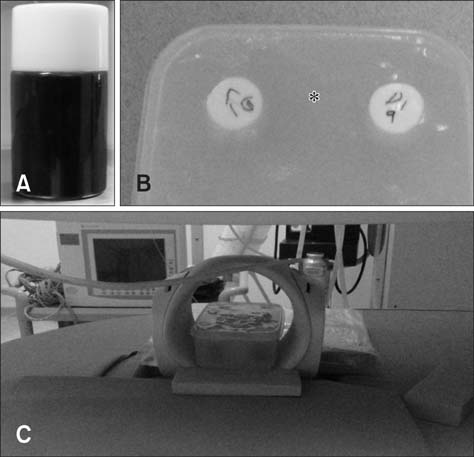
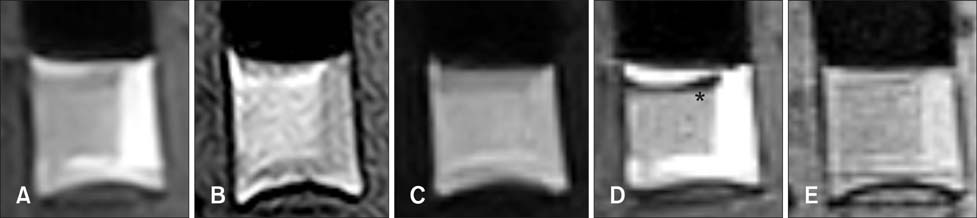
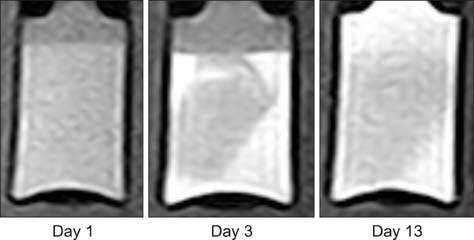
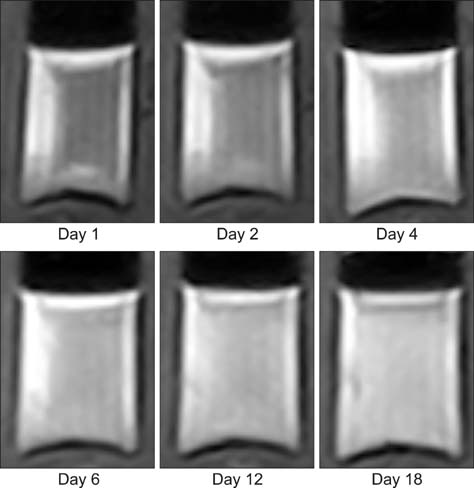
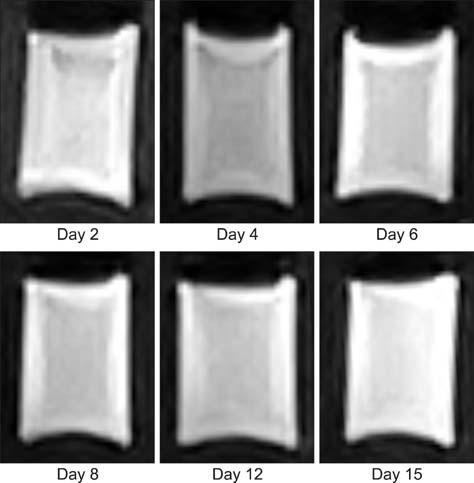
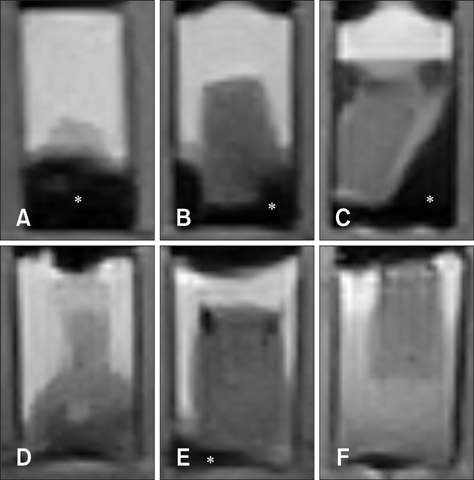
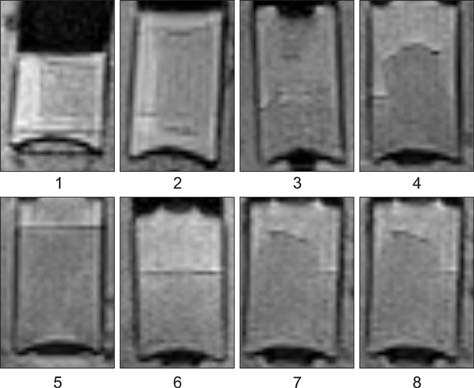
- This study was conducted to assess time-sensitive magnetic resonance (MR) changes in canine blood using low-field MR. Arterial and venous blood samples were collected from eight healthy beagle dogs. Samples were placed in 5-mL tubes and imaged within 3 hours of collection at 1 day intervals from day 1 to day 30. The following sequences were used: T1-weighted (T1W), T2-weighted (T2W), fluid-attenuated inversion recovery (FLAIR), short tau inversion recovery (STIR), and T2-star gradient-echo (T2*-GRE). Visual comparison of the images revealed that four relatively homogenous blood clots and twelve heterogeneous blood clots developed. The margination of the clot and plasma changed significantly on day 2 and day 13. On day 2, heterogeneous blood clots were differentiated into 2 to 3 signal layers in the T2W, T1W, and especially the STIR images. Hypointense signal layers were also detected in the blood clots in STIR images, which have T2 hypo, FLAIR hypo, and T1 hyper intense signals. In all images, these signal layers remained relatively unchanged until day 13. Overall, the results suggest that hematomas are complex on low-field MRI. Accordingly, it may not be feasible to accurately characterize hemorrhages and predict clot age based on low-field MRI.
Keyword
MeSH Terms
Figure
Reference
-
1. Atlas SW, Mark AS, Grossman RI, Gomori JM. Intracranial hemorrhage: gradient-echo MR imaging at 1.5 T. Comparison with spin-echo imaging and clinical applications. Radiology. 1988; 168:803–807.
Article2. Bakshi R, Kamran S, Kinkel PR, Bates VE, Mechtler LL, Janardhan V, Belani SL, Kinkel WR. Fluid-attenuated inversion-recovery MR imaging in acute and subacute cerebral intraventricular hemorrhage. AJNR Am J Neuroradiol. 1999; 20:629–636.3. Bellon EM, Haacke EM, Coleman PE, Sacco DC, Steiger DA, Gangarosa RE. MR artifacts: a review. AJR Am Roentgenol. 1986; 147:1271–1281.
Article4. Bradley WG Jr. MR appearance of hemorrhage in the brain. Radiology. 1993; 189:15–26.
Article5. Brooks RA, Di Chiro G, Patronas N. MR imaging of cerebral hematomas at different field strengths: theory and applications. J Comput Assist Tomogr. 1989; 13:194–206.6. Cohen MD, McGuire W, Cory DA, Smith JA. Society for Pediatric Radiology John Caffey Award. MR appearance of blood and blood products: an in vitro study. AJR Am J Roentgenol. 1986; 146:1293–1297.
Article7. Dürr HR, Lienemann A, Stäbler A, Küehne J, Refior HJ. MRI of posttraumatic cyst-like lesions of bone after a greenstick fracture. Eur Radiol. 1997; 7:1218–1220.
Article8. Farahani K, Sinha U, Sinha S, Chiu LC, Lufkin RB. Effect of field strength on susceptibility artifacts in magnetic resonance imaging. Comput Med Imaging Graph. 1990; 14:409–413.
Article9. Fulkerson CV, Young BD, Jackson ND, Porter B, Levine JM. MRI Characteristics of cerebral microbleeds in four dogs. Vet Radiol Ultrasound. 2012; 53:389–393.
Article10. Gavin PR, Bagley RS. Practical Small Animal MRI. 1st ed. Ames: Wiley-Blackwell;2009. p. 60.11. Hodshon AW, Hecht S, Thomas WB. Use of the T2*‐ weighted gradient recalled echo sequence for magnetic resonance imaging of the canine and feline brain. Vet Radiol Ultrasound. 2014; 55:599–606.
Article12. Konar M, Lang J. Pros and cons of low-field magnetic resonance imaging in veterinary practice. Vet Radiol Ultrasound. 2011; 52:Suppl 1. S5–S14.
Article13. Mateo I, Lorenzo V, Foradada L, Muñoz A. Clinical, pathologic, and magnetic resonance imaging characteristics of canine disc extrusion accompanied by epidural hemorrhage or inflammation. Vet Radiol Ultrasound. 2011; 52:17–24.
Article14. Pang KK, Tsai YS, Chang HC, Hsu KN. Methemoglobin suppression in a 0.3 Tesla magnet: an in vitro and in vivo study. Acad Radiol. 2010; 17:624–627.15. Parizel PM, Makkat S, Van Miert E, Van Goethem JW, van den Hauwe L, De Schepper AM. Intracranial hemorrhage: principles of CT and MRI interpretation. Eur Radiol. 2001; 11:1770–1783.
Article16. Swensen SJ, Keller PL, Berquist TH, McLeod RA, Stephens DH. Magnetic resonance imaging of hemorrhage. AJR Am J Roentgenol. 1985; 145:921–927.
Article17. Taber KH, Hayman LA, Herrick RC, Kirkpatrick JB. Importance of clot structure in gradient‐echo magnetic resonance imaging of hematoma. J Magn Reson Imaging. 1996; 6:878–883.
Article18. Tamura S, Tamura Y, Tsuka T, Uchida K. Sequential magnetic resonance imaging of an intracranial hematoma in a dog. Vet Radiol Ultrasound. 2006; 47:142–144.
Article19. Thomas WB, Adams WH, McGavin MD, Gompf RE. Magnetic resonance imaging appearance of intracranial hemorrhage secondary to cerebral vascular malformation in a dog. Vet Radiol Ultrasound. 1997; 38:371–375.
Article20. Tidwell AS, Specht A, Blaeser L, Kent M. Magnetic resonance imaging features of extradural hematomas associated with intervertebral disc herniation in a dog. Vet Radiol Ultrasound. 2002; 43:319–324.
Article21. Unger EC, Glazer HS, Lee JK, Ling D. MRI of extracranial hematomas: preliminary observations. AJR Am J Roentgenol. 1986; 146:403–407.
Article22. Weingarten K, Zimmerman RD, Deo-Narine V, Markisz J, Cahill PT, Deck MD. MR imaging of acute intracranial hemorrhage: findings on sequential spin-echo and gradient-echo images in a dog model. AJNR Am J Neuroradiol. 1991; 12:457–467.23. Zimmerman RD, Heier LA, Snow RB, Liu DP, Kelly AB, Deck MD. Acute intracranial hemorrhage: intensity changes on sequential MR scans at 0.5 T. AJR Am J Roentgenol. 1988; 150:651–661.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Nitric Oxide in the Relaxation of Canine Corpus Cavernosum Smooth Muscle
- Diagnostic imaging features of normal anal sacs in dogs and cats
- Development and Feasibility Study for Phase Contrast MR Angiography at Low Tesla Open-MRI System
- Treatment Planning Correction Using MRI in the Radiotherapy of Cervical Cancer
- Higher Lesion Detection by 3.0T MRI in Patient with Transient Global Amnesia