Ann Lab Med.
2015 Jan;35(1):28-34. 10.3343/alm.2015.35.1.28.
Flow Cytometric White Blood Cell Differential Using CytoDiff is Excellent for Counting Blasts
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea. hankja@catholic.ac.kr
- KMID: 2363144
- DOI: http://doi.org/10.3343/alm.2015.35.1.28
Abstract
- BACKGROUND
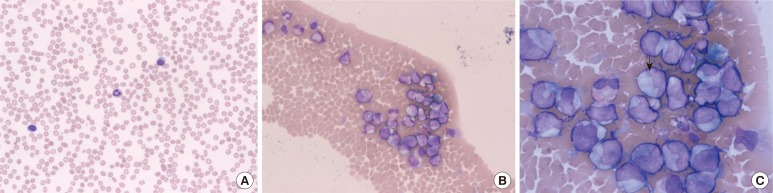
The usefulness of the CytoDiff flow cytometric system (Beckman Coulter, USA) has been studied in various conditions, but its performance including rapidity in detecting and counting blasts, the most significant abnormal cells in the peripheral blood, has not been well evaluated. The objective of this study was to evaluate the performance of the CytoDiff differential counting method in challenging samples with blasts.
METHODS
In total, 815 blood samples were analyzed. Samples flagged as "blasts" or "variant lymphocytes" and showing <10% blasts by manual counts were included. In total, 322 samples showed blasts on manual counts, ranging from 0.5% to 99%. The CytoDiff method was performed by flow cytometry (FC500; Beckman Coulter, USA) with a pre-mixed CytoDiff reagent and analyzing software (CytoDiff CXP 2.0; Beckman Coulter).
RESULTS
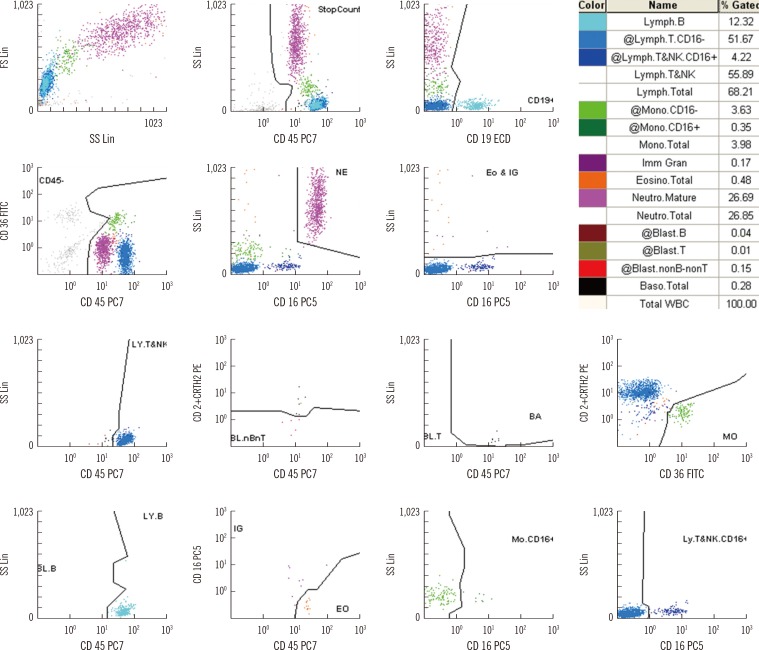
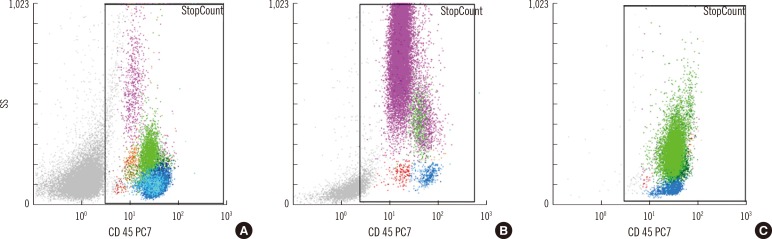
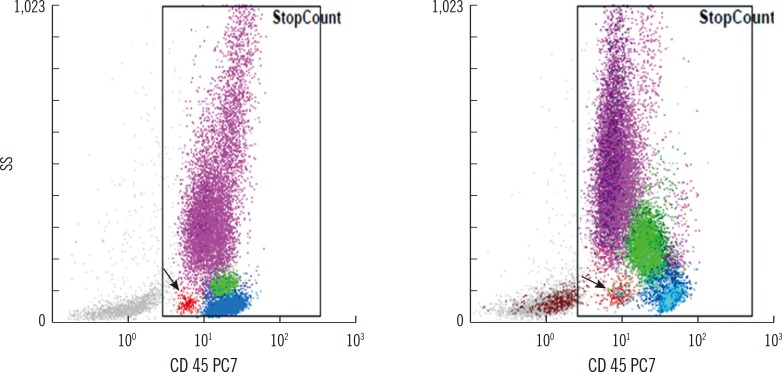
The average time required to analyze 20 samples was approximately 60 min for manual counts, and the hands-on time for the CytoDiff method was 15 min. The correlation between the CytoDiff and manual counts was good (r>0.8) for neutrophils and lymphocytes but poor (r<0.8) for other cells. When the cutoff value of the CytoDiff blast count was set at 1%, the sensitivity was 94.4% (95% CI; 91.2-96.6) and specificity was 91.9% (95% CI; 89.0-94.1). The positive predictive value was 88.4% (95% CI; 84.4-91.5) (304/344 cases) and negative predictive value was 96.2% (95% CI; 93.9-97.7) (453/471 cases). The CytoDiff blast counts correlated well to the manual counts (r=0.9223).
CONCLUSIONS
The CytoDiff method is a specific, sensitive, and rapid method for counting blasts. A cutoff value of 1% of at least 1 type of blast is recommended for positive CytoDiff blast counts.
Keyword
MeSH Terms
Figure
Reference
-
1. Simson E, Groner W. The state of the art for the automated WBC differential. Part 1: analytic performance. Lab Hematol. 1994; 1:13–22.2. Pierre RV. Peripheral blood film review. The demise of the eyecount leukocyte differential. Clin Lab Med. 2002; 22:279–297. PMID: 11933579.
Article3. Barnes PW, McFadden SL, Machin SJ, Simson E. International consensus group for hematology. The international consensus groupfor hematology review: suggested criteria for action following automated CBC and WBC differential analysis. Lab Hematol. 2005; 11:83–90. PMID: 16024331.4. Novis DA, Walsh M, Wilkinson D, St Louis M, Ben-Ezra J. Laboratory productivity and the rate of manual peripheral blood smear review: a College of American Pathologists Q-Probes study of 95,141 complete blood count determinations performed in 263 institutions. Arch Pathol Lab Med. 2006; 130:596–601. PMID: 16683868.
Article5. Guerti K, Vertessen F, Daniëls L, Van Der Planken M. Performance evaluation of the PENTRA 60C+ automated hematology analyzer and comparison with the ADVIA 2120. Int J Lab Hematol. 2009; 31:132–141. PMID: 19267810.6. Eilertsen H, Vøllestad NK, Hagve TA. The usefulness of blast flags on the Sysmex XE-5000 is questionable. Am J Clin Pathol. 2013; 139:633–640. PMID: 23596115.
Article7. Briggs C, Longair I, Slavik M, Thwaite K, Mills R, Thavaraja V, et al. Can automated blood film analysis replace the manual differential? Anevaluation of the CellaVision DM96 automated image analysis system. Int J Lab Hematol. 2009; 31:48–60. PMID: 18177438.8. Kratz A, Bengtsson HI, Casey JE, Keefe JM, Beatrice GH, Grzybek DY, et al. Performance evaluation of the CellaVision DM96 system: WBC differentials by automated digital image analysis supported by an artificial neural network. Am J Clin Pathol. 2005; 124:770–781. PMID: 16203273.9. Rümke CL. Imprecision of ratio-derived differential leukocyte counts. Blood Cells. 1985; 11:311–315. PMID: 3834968.10. Roussel M, Davis BH, Fest T, Wood BL. International Council for Standardization in Hematology (ICSH). Toward a reference method for leukocyte differential counts in blood: comparison of three flow cytometric candidate methods. Cytometry A. 2012; 81:973–982. PMID: 22736499.
Article11. Kulkarni S, Ghosh SP, Hauer-Jensen M, Kumar KS. Hematological targets of radiation damage. Curr Drug Targets. 2010; 11:1375–1385. PMID: 20583980.
Article12. Saloustros E, Tryfonidis K, Georgoulias V. Prophylactic and therapeutic strategies in chemotherapy-induced neutropenia. Expert Opin Pharmacother. 2011; 12:851–863. PMID: 21254862.
Article13. Fuentes-Arderiu X, Dot-Bach D. Measurement uncertainty in manual differential leukocyte counting. Clin Chem Lab Med. 2009; 47:112–115. PMID: 19072029.
Article14. Faucher JL, Lacronique-Gazaille C, Frébet E, Trimoreau F, Donnard M, Bordessoule D, et al. "6 markers/5 colors" extended white blood cell differential by flow cytometry. Cytometry A. 2007; 71:934–944. PMID: 17879238.
Article15. Jo Y, Kim SH, Koh K, Park J, Shim YB, Lim J, et al. Reliable, accurate determination of the leukocyte differential of leukopenic samples by using Hematoflow method. Korean J Lab Med. 2011; 31:131–137. PMID: 21779183.
Article16. Park SH, Park BG, Park CJ, Kim S, Kim DH, Jang S, et al. An extended leukocyte differential count (16 types of circulating leukocytes) using the CytoDiff flow cytometric system can provide information for the discrimination of sepsis severity and prediction of outcome in sepsis patients. Cytometry B Clin Cytom. 2014; 86:244–256. PMID: 24002800.
Article17. Park BG, Park CJ, Yoon CH, Jang S, Chi HS, Ryu MH, et al. The extended leukocyte differential count using the Cytodiff flow cytometric system reveals that higher CD16+ cytotoxic NK+T lymphocyte levels predict superior survival outcomes in patients with metastatic carcinoma. Cytometry B Clin Cytom. 2013; 84:202–204. PMID: 23281059.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Flow Cytometric White Blood Cell Differential Using CytoDiff is Excellent for Counting Blasts
- Reliable, Accurate Determination of the Leukocyte Differential of Leukopenic Samples by Using Hematoflow Method
- White blood cell differential counts in severely leukopenic samples: a comparative analysis of different solutions available in modern laboratory hematology
- Development of a Novel Flow Cytometry-Based System for White Blood Cell Differential Counts: 10-color LeukoDiff
- Flow Cytometric Method for Counting Residual Leukocytes