J Pathol Transl Med.
2016 Nov;50(6):411-418. 10.4132/jptm.2016.08.08.
Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csikpark@amc.seoul.kr
- KMID: 2361988
- DOI: http://doi.org/10.4132/jptm.2016.08.08
Abstract
- Immunohistochemistry (IHC) is an important auxiliary method for pathologists in routine diagnostic work as well as in basic and clinical research including exploration of biomarkers, as IHC allows confirmation of target molecule expressions in the context of microenvironment. Although there has been a considerable progress in automation and standardization of IHC, there are still many things to be considered in proper optimization and appropriate interpretation. In this review, we aim to provide possible pitfalls and useful tips for practicing pathologists and residents in pathology training. First, general procedure of IHC is summarized, followed by pitfalls and tips in each step and a summary of troubleshooting. Second, ways to an accurate interpretation of IHC are discussed, with introduction to general quantification and analysis methods. This review is not intended to provide complete information on IHC, but to be used as a basic reference for practice and publication.
MeSH Terms
Figure
Reference
-
1. Schacht V, Kern JS. Basics of immunohistochemistry. J Invest Dermatol. 2015; 135:e30.
Article2. O’Hurley G, Sjostedt E, Rahman A, et al. Garbage in, garbage out: a critical evaluation of strategies used for validation of immunohistochemical biomarkers. Mol Oncol. 2014; 8:783–98.3. Gustavson MD, Bourke-Martin B, Reilly D, et al. Standardization of HER2 immunohistochemistry in breast cancer by automated quantitative analysis. Arch Pathol Lab Med. 2009; 133:1413–9.
Article4. Kumar V, Abbas AK, Fausto N. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia: Elsevier Saunders;2005.5. Pekmezci M, Szpaderska A, Osipo C, Ers¸ahin Ç. The Effect of cold ischemia time and/or formalin fixation on estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 results in breast carcinoma. Patholog Res Int. 2012; 2012:947041.
Article6. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007; 131:18–43.7. Cross SS, Start RD, Smith JH. Does delay in fixation affect the number of mitotic figures in processed tissue? J Clin Pathol. 1990; 43:597–9.
Article8. Engel KB, Moore HM. Effects of preanalytical variables on the detection of proteins by immunohistochemistry in formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. 2011; 135:537–43.
Article9. Grizzle WE. Special symposium: fixation and tissue processing models. Biotech Histochem. 2009; 84:185–93.
Article10. Wester K, Wahlund E, Sundström C, et al. Paraffin section storage and immunohistochemistry: effects of time, temperature, fixation, and retrieval protocol with emphasis on p53 protein and MIB1 antigen. Appl Immunohistochem Mol Morphol. 2000; 8:61–70.11. Prioleau J, Schnitt SJ. p53 antigen loss in stored paraffin slides. N Engl J Med. 1995; 332:1521–2.
Article12. Xie R, Chung JY, Ylaya K, et al. Factors influencing the degradation of archival formalin-fixed paraffin-embedded tissue sections. J Histochem Cytochem. 2011; 59:356–65.
Article13. Fox CH, Johnson FB, Whiting J, Roller PP. Formaldehyde fixation. J Histochem Cytochem. 1985; 33:845–53.
Article14. Shi SR, Liu C, Taylor CR. Standardization of immunohistochemistry for formalin-fixed, paraffin-embedded tissue sections based on the antigen-retrieval technique: from experiments to hypothesis. J Histochem Cytochem. 2007; 55:105–9.
Article15. Vogt RF Jr, Phillips DL, Henderson LO, Whitfield W, Spierto FW. Quantitative differences among various proteins as blocking agents for ELISA microtiter plates. J Immunol Methods. 1987; 101:43–50.
Article16. Radulescu RT, Boenisch T. Blocking endogenous peroxidases: a cautionary note for immunohistochemistry. J Cell Mol Med. 2007; 11:1419.17. Garba MT, Marie PJ. Alkaline phosphatase inhibition by levamisole prevents 1,25-dihydroxyvitamin D3-stimulated bone mineralization in the mouse. Calcif Tissue Int. 1986; 38:296–302.
Article18. Taylor CR, Levenson RM. Quantification of immunohistochemistry: issues concerning methods, utility and semiquantitative assessment II. Histopathology. 2006; 49:411–24.19. Skliris GP, Rowan BG, Al-Dhaheri M, et al. Immunohistochemical validation of multiple phospho-specific epitopes for estrogen receptor alpha (ERalpha) in tissue microarrays of ERalpha positive human breast carcinomas. Breast Cancer Res Treat. 2009; 118:443–53.20. Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000; 19:113–32.
Article21. Hou L, Tang Y, Xu M, Gao Z, Tang D. Tyramine-based enzymatic conjugate repeats for ultrasensitive immunoassay accompanying tyramine signal amplification with enzymatic biocatalytic precipitation. Anal Chem. 2014; 86:8352–8.
Article22. Stefanovic´ D, Stefanovic´ M, Nikin Z. Romanowsky-Giemsa as a counterstain for immunohistochemistry: optimizing a traditional reagent. Biotech Histochem. 2013; 88:329–35.23. Dabbs DJ. Diagnostic immunohistochemistry: theranostic and genomic applications. 4th ed. Philadelphia: Elsevier Saunders;2014.24. Tzankov A, Zlobec I, Went P, Robl H, Hoeller S, Dirnhofer S. Prognostic immunophenotypic biomarker studies in diffuse large B cell lymphoma with special emphasis on rational determination of cut-off scores. Leuk Lymphoma. 2010; 51:199–212.
Article25. Shackelford C, Long G, Wolf J, Okerberg C, Herbert R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002; 30:93–6.
Article26. Thoolen B, Maronpot RR, Harada T, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010; 38(7 suppl):5S–81S.
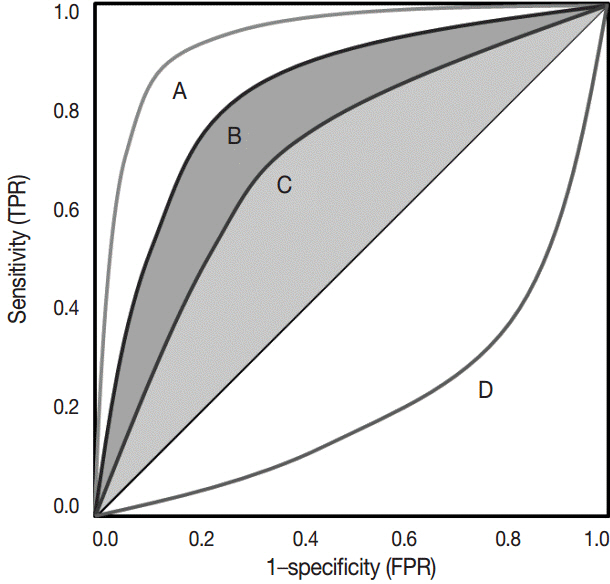
Article27. Hanley JA. Receiver operating characteristic (ROC) methodology: the state of the art. Crit Rev Diagn Imaging. 1989; 29:307–35.28. Lusted LB. Signal detectability and medical decision-making. Science. 1971; 171:1217–9.
Article29. Søreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009; 62:1–5.30. Zlobec I, Steele R, Terracciano L, Jass JR, Lugli A. Selecting immunohistochemical cut-off scores for novel biomarkers of progression and survival in colorectal cancer. J Clin Pathol. 2007; 60:1112–6.
Article31. Yaziji H, Barry T. Diagnostic Immunohistochemistry: what can go wrong? Adv Anat Pathol. 2006; 13:238–46.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical Application to the Pathologic Diagnosis of Lung Cancer
- The role of transjugular intrahepatic portosystemic shunt in patients with portal hypertension: Advantages and pitfalls
- The Tips and Pitfalls of Meniscus Allograft Transplantation
- Book Review: Pearls and Pitfalls in Head and Neck Surgery: Practical Tips to Minimize Complications
- Hepatocellular adenomas: recent updates