J Korean Med Sci.
2015 Dec;30(12):1793-1799. 10.3346/jkms.2015.30.12.1793.
Prognostic Inflammation Score in Surgical Patients with Colorectal Cancer
- Affiliations
-
- 1Department of Surgery, Konkuk University School of Medicine, Seoul, Korea. recto@kuh.ac.kr
- KMID: 2359963
- DOI: http://doi.org/10.3346/jkms.2015.30.12.1793
Abstract
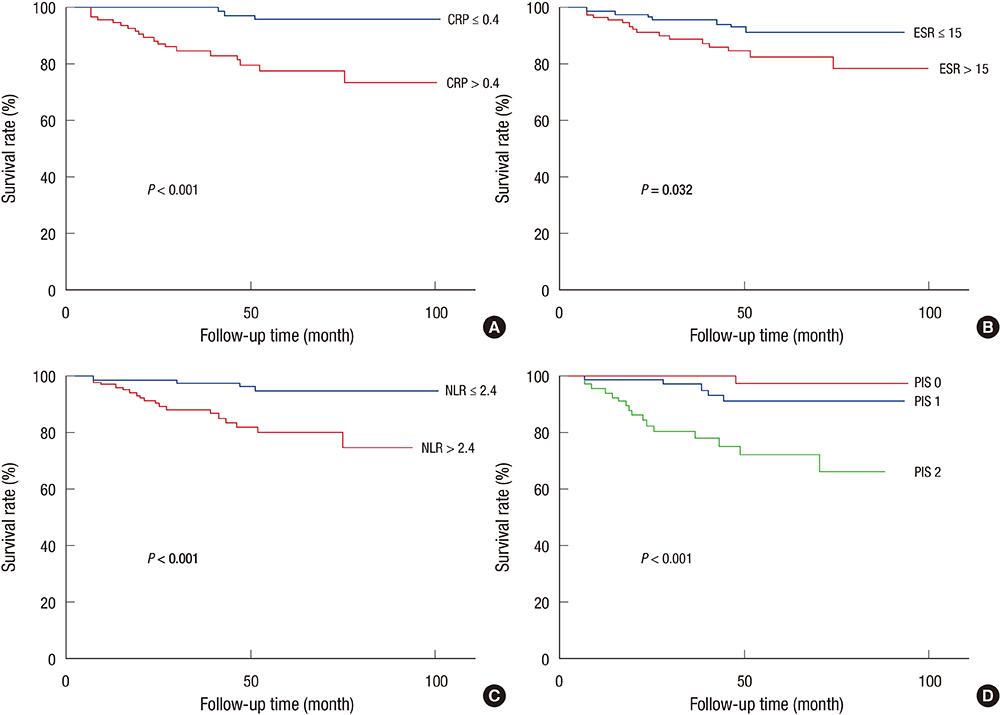
- Several inflammatory markers have been investigated as prognostic parameters in a variety of cancer population with mostly favorable results. This study aimed to verify the significance of common inflammatory markers as prognostic variables and assess whether a selective combination of them as prognostic inflammation score (PIS) could further improve their prognostic values in surgical patients with colorectal cancer (CRC). A total of 265 patients who had undergone curative resection of CRC were reviewed retrospectively. Preoperative levels of inflammatory markers such as serum C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and neutrophil/lymphocyte ratio (NLR) were assessed by uni- and multivariate survival analysis with disease-free (DFS) and disease-specific survival (DSS). PIS was constructed with a selective combination of inflammatory markers which were independently significant. On univariate analysis, CRP, ESR, and NLR were significantly associated with DFS and DSS. On multivariate analysis, CRP and NLR were independently significant prognostic variables for DSS and DFS respectively (P=0.013, P=0.021). When PIS was constructed with combination of CRP and NLR, it was independently and significantly associated with both DFS and DSS (P=0.006, P=0.010). Furthermore, PIS was superior to CRP for DSS (HR=15.679 vs. HR=5.183), and NLR for DFS in terms of prognosticating power (HR=4.894 vs. HR=2.687). When PIS is constructed with combination of CRP and NLR, it is a potentially significant prognostic variable associated with poor survival regardless pathologic prognostic variables in patients with CRC after curative resection.
Keyword
MeSH Terms
Figure
Reference
-
1. Mazhar D, Ngan S. C-reactive protein and colorectal cancer. QJM. 2006; 99:555–559.2. Sox HC Jr, Liang MH. The erythrocyte sedimentation rate. Guidelines for rational use. Ann Intern Med. 1986; 104:515–523.3. Wyllie DH, Bowler IC, Peto TE. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol. 2004; 57:950–955.4. Zahorec R. Ratio of neutrophil to lymphocyte counts: rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001; 102:5–14.5. Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004; 90:1704–1706.6. Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010; 17:52–58.7. Siemes C, Visser LE, Coebergh JW, Splinter TA, Witteman JC, Uitterlinden AG, Hofman A, Pols HA, Stricker BH. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006; 24:5216–5222.8. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003; 90:215–219.9. Nozoe T, Mori E, Takahashi I, Ezaki T. Preoperative elevation of serum C-reactive protein as an independent prognostic indicator of colorectal carcinoma. Surg Today. 2008; 38:597–602.10. Sengupta S, Lohse CM, Cheville JC, Leibovich BC, Thompson RH, Webster WS, Frank I, Zincke H, Blute ML, Kwon ED. The preoperative erythrocyte sedimentation rate is an independent prognostic factor in renal cell carcinoma. Cancer. 2006; 106:304–312.11. Choi ES, Kim HS, Han I. Elevated preoperative systemic inflammatory markers predict poor outcome in localized soft tissue sarcoma. Ann Surg Oncol. 2014; 21:778–785.12. Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non-small-cell lung cancer: an analysis of Japan Multinational Trial Organisation LC00-03. Eur J Cancer. 2009; 45:1950–1958.13. Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011; 104:1288–1295.14. Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009; 197:466–472.15. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–899.16. Nozoe T, Kimura Y, Ishida M, Saeki H, Korenaga D, Sugimachi K. Correlation of pre-operative nutritional condition with post-operative complications in surgical treatment for oesophageal carcinoma. Eur J Surg Oncol. 2002; 28:396–400.17. Costenbader KH, Chibnik LB, Schur PH. Discordance between erythrocyte sedimentation rate and C-reactive protein measurements: clinical significance. Clin Exp Rheumatol. 2007; 25:746–749.18. Colombet I, Pouchot J, Kronz V, Hanras X, Capron L, Durieux P, Wyplosz B. Agreement between erythrocyte sedimentation rate and C-reactive protein in hospital practice. Am J Med. 2010; 123:863.e7–863.e13.19. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003; 111:1805–1812.20. Jurado RL. Why shouldn't we determine the erythrocyte sedimentation rate? Clin Infect Dis. 2001; 33:548–549.21. Hansson LO, Carlsson I, Hansson E, Hovelius B, Svensson P, Tryding N. Measurement of C-reactive protein and the erythrocyte sedimentation rate in general practice. Scand J Prim Health Care. 1995; 13:39–45.22. Holub M, Beran O, Kaspříková N, Chalupa P. Neutrophil to lymphocyte count ratio as a biomarker of bacterial infections. Cent Eur J Med. 2012; 7:258–261.23. Okano K, Maeba T, Moroguchi A, Ishimura K, Karasawa Y, Izuishi K, Goda F, Usuki H, Wakabayashi H, Maeta H. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol. 2003; 82:28–33.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammation-based score (Glasgow prognostic score) as an independent prognostic factor in colorectal cancer patients
- Prognostic Factors Associated with Surgical Mortality Conferred by Emergency Operation in Colorectal Cancer
- Crossroad between inflammation and carcinogenesis in colon
- Preoperative thrombocytosis predicts prognosis in stage II colorectal cancer patients
- Multivariate analysis of prognostic factors in colorectal cancer patients: significance of lymph node metastasis as a prognostic factor in colorectal cancer