J Rheum Dis.
2016 Oct;23(5):297-303. 10.4078/jrd.2016.23.5.297.
Chemokine (C-X-C Motif) Ligand 1 (CXCL1) Expression in the Minor Salivary Glands of Sjögren's Syndrome Patients
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea. shinseok@chonnam.ac.kr
- 2Department of Pathology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Ophthalmology, Chonnam National University Hospital, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 2356484
- DOI: http://doi.org/10.4078/jrd.2016.23.5.297
Abstract
OBJECTIVE
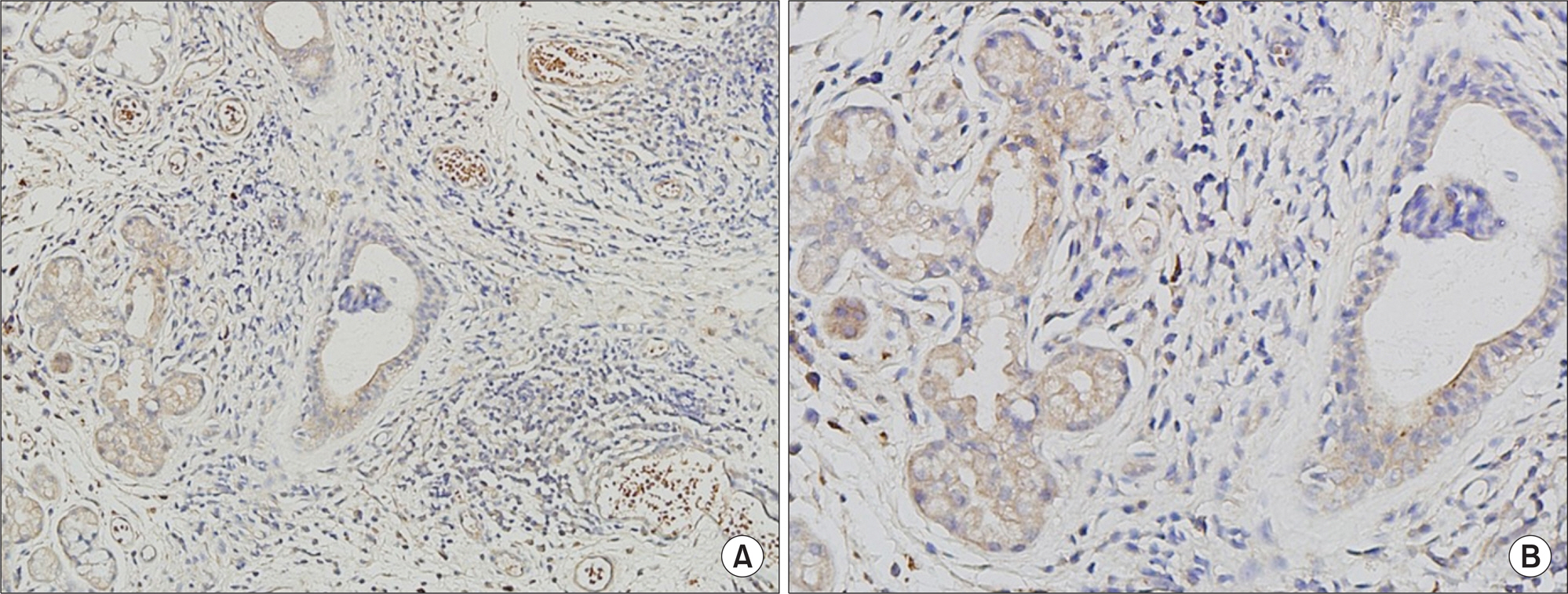
To evaluate the laboratory and clinical manifestations of Sjögren's syndrome (SS) association with chemokine (C-X-C motif) ligand 1 (CXCL1) expression in the ductal and acinar salivary gland epithelial cells (SGEC) of the minor salivary glands.
METHODS
The sociodemographic data of 106 SS patients was obtained, and the glandular and extraglandular manifestations of the disease documented. The minor salivary glands were biopsied and the laboratory findings analyzed. European League Against Rheumatism SS disease activity index (ESSDAI) and SS disease damage index (SSDDI) scores were obtained during biopsy. An immunohistochemical approach was used to define the expression of CXCL1 in the salivary glands.
RESULTS
Of 106 patients, the minor salivary glands of 22 patients (20.7%) stained positively for CXCL1. Such CXCL1-positive patients exhibited higher ESSDAI scores at the time of biopsy than the CXCL1-negative patients (3.86±2.27 vs. 2.64±1.62, p=0.015). Lymphadenopathy was more frequently observed in CXCL1-positive patients, compared with CXCL1-negative patients (31.8% vs. 9.5%, p=0.014). No differences between groups were identified in terms of sociodemographic characteristics, laboratory data, or the extent of the glandular manifestation of SS.
CONCLUSION
The expression of CXCL1 within the ductal and acinar SGEC of SS patients is associated with lymphadenopathy and elevated clinical disease activity. CXCL1 may play an important role in the disease activity and prognosis of SS.
Keyword
MeSH Terms
Figure
Reference
-
1. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004; 164:1275–84.
Article2. Hansen A, Lipsky PE, Dörner T. Immunopathogenesis of primary Sjögren's syndrome: implications for disease management and therapy. Curr Opin Rheumatol. 2005; 17:558–65.
Article3. Manoussakis MN, Moutsopoulos HM. Sjögren's syndrome: autoimmune epithelitis. Baillieres Best Pract Res Clin Rheumatol. 2000; 14:73–95.
Article4. Barone F, Bombardieri M, Rosado MM, Morgan PR, Challacombe SJ, De Vita S, et al. CXCL13, CCL21, and CXCL12 expression in salivary glands of patients with Sjogren's syndrome and MALT lymphoma: association with reactive and malignant areas of lymphoid organization. J Immunol. 2008; 180:5130–40.5. Bourazopoulou E, Kapsogeorgou EK, Routsias JG, Manoussakis MN, Moutsopoulos HM, Tzioufas AG. Functional expression of the alpha 2-macroglobulin receptor CD91 in salivary gland epithelial cells. J Autoimmun. 2009; 33:141–6.
Article6. Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjögren's syndrome. J Immunol. 1994; 152:5532–9.7. Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006; 54:2295–9.
Article8. Tsunawaki S, Nakamura S, Ohyama Y, Sasaki M, Ikebe-Hiroki A, Hiraki A, et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjögren's syndrome. J Rheumatol. 2002; 29:1884–96.9. Kawakami A, Nakashima K, Tamai M, Nakamura H, Iwanaga N, Fujikawa K, et al. Toll-like receptor in salivary glands from patients with Sjögren's syndrome: functional analysis by human salivary gland cell line. J Rheumatol. 2007; 34:1019–26.10. Dimitriou ID, Kapsogeorgou EK, Moutsopoulos HM, Manoussakis MN. CD40 on salivary gland epithelial cells: high constitutive expression by cultured cells from Sjögren's syndrome patients indicating their intrinsic activation. Clin Exp Immunol. 2002; 127:386–92.
Article11. Ittah M, Miceli-Richard C, Gottenberg JE, Sellam J, Eid P, Lebon P, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur J Immunol. 2008; 38:1058–64.
Article12. Manoussakis MN, Dimitriou ID, Kapsogeorgou EK, Xanthou G, Paikos S, Polihronis M, et al. Expression of B7 costimulatory molecules by salivary gland epithelial cells in patients with Sjögren's syndrome. Arthritis Rheum. 1999; 42:229–39.
Article13. Spachidou MP, Bourazopoulou E, Maratheftis CI, Kapsogeorgou EK, Moutsopoulos HM, Tzioufas AG, et al. Expression of functional Toll-like receptors by salivary gland epithelial cells: increased mRNA expression in cells derived from patients with primary Sjögren's syndrome. Clin Exp Immunol. 2007; 147:497–503.
Article14. Ping L, Ogawa N, Sugai S. Novel role of CD40 in Fas-de-pendent apoptosis of cultured salivary epithelial cells from patients with Sjögren's syndrome. Arthritis Rheum. 2005; 52:573–81.
Article15. Richmond A, Thomas HG. Purification of melanoma growth stimulatory activity. J Cell Physiol. 1986; 129:375–84.
Article16. Iida N, Grotendorst GR. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol Cell Biol. 1990; 10:5596–9.
Article17. Becker S, Quay J, Koren HS, Haskill JS. Constitutive and stimulated MCP-1, GRO alpha, beta, and gamma expression in human airway epithelium and bronchoalveolar macrophages. Am J Physiol. 1994; 266:L278–86.
Article18. Moser B, Clark-Lewis I, Zwahlen R, Baggiolini M. Neutro-phil-activating properties of the melanoma growth-stimulatory activity. J Exp Med. 1990; 171:1797–802.
Article19. Vries MH, Wagenaar A, Verbruggen SE, Molin DG, Dijkgraaf I, Hackeng TH, et al. CXCL1 promotes arterio-genesis through enhanced monocyte recruitment into the peri-collateral space. Angiogenesis. 2015; 18:163–71.
Article20. Haghnegahdar H, Du J, Wang D, Strieter RM, Burdick MD, Nanney LB, et al. The tumorigenic and angiogenic effects of MGSA/GRO proteins in melanoma. J Leukoc Biol. 2000; 67:53–62.
Article21. Bechara C, Chai H, Lin PH, Yao Q, Chen C. Growth related oncogene-alpha (GRO-alpha): roles in atherosclerosis, angiogenesis and other inflammatory conditions. Med Sci Monit. 2007; 13:RA87–90.22. Jacobs JP, Ortiz-Lopez A, Campbell JJ, Gerard CJ, Mathis D, Benoist C. Deficiency of CXCR2, but not other chemokine receptors, attenuates autoantibody-mediated arthritis in a murine model. Arthritis Rheum. 2010; 62:1921–32.23. Furuse S, Fujii H, Kaburagi Y, Fujimoto M, Hasegawa M, Takehara K, et al. Serum concentrations of the CXC chemokines interleukin 8 and growth-regulated oncogene-alpha are elevated in patients with systemic sclerosis. J Rheumatol. 2003; 30:1524–8.24. Lisi S, Sisto M, Lofrumento DD, D'Amore M, De Lucro R, Ribatti D. A potential role of the GRO-α/CXCR2 system in Sjögren's syndrome: regulatory effects of proinflammatory cytokines. Histochem Cell Biol. 2013; 139:371–9.
Article25. Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61:554–8.26. Daniels TE, Whitcher JP. Association of patterns of labial salivary gland inflammation with keratoconjunctivitis sicca. Analysis of 618 patients with suspected Sjögren's syndrome. Arthritis Rheum. 1994; 37:869–77.27. Seror R, Ravaud P, Bowman SJ, Baron G, Tzioufas A, Theander E, et al. EULAR Sjogren's syndrome disease activity index: development of a consensus systemic disease activity index for primary Sjogren's syndrome. Ann Rheum Dis. 2010; 69:1103–9.28. Vitali C, Palombi G, Baldini C, Benucci M, Bombardieri S, Covelli M, et al. Sjögren's Syndrome Disease Damage Index and disease activity index: scoring systems for the assessment of disease damage and disease activity in Sjögren's syndrome, derived from an analysis of a cohort of Italian patients. Arthritis Rheum. 2007; 56:2223–31.
Article29. Sisto M, Lisi S. New insights into ADAMs regulation of the GRO-α/CXCR2 system: focus on Sjögren's syndrome. Int Rev Immunol. 2015; 34:486–99.
Article30. Jinquan T, Frydenberg J, Mukaida N, Bonde J, Larsen CG, Matsushima K, et al. Recombinant human growth-regu-lated oncogene-alpha induces T lymphocyte chemotaxis. A process regulated via IL-8 receptors by IFN-gamma, TNF-alpha, IL-4, IL-10, and IL-13. J Immunol. 1995; 155:5359–68.31. Schwartz D, Andalibi A, Chaverri-Almada L, Berliner JA, Kirchgessner T, Fang ZT, et al. Role of the GRO family of chemokines in monocyte adhesion to MM-LDL-stimulated endothelium. J Clin Invest. 1994; 94:1968–73.
Article32. Sisto M, Lisi S, Lofrumento DD, D'Amore M, Frassanito MA, Ribatti D. Sjögren's syndrome pathological neovascularization is regulated by VEGF-A-stimulated TACE-de-pendent crosstalk between VEGFR2 and NF-κ B. Genes Immun. 2012; 13:411–20.33. Sisto M, Lisi S, Lofrumento DD, D'Amore M, Ribatti D. Neuropilin-1 is upregulated in Sjögren's syndrome and contributes to pathological neovascularization. Histochem Cell Biol. 2012; 137:669–77.
Article34. Sisto M, Lisi S, Ingravallo G, Lofrumento DD, D'Amore M, Ribatti D. Neovascularization is prominent in the chronic inflammatory lesions of Sjögren's syndrome. Int J Exp Pathol. 2014; 95:131–7.
Article35. Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004; 4:953–64.
Article36. Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006; 43(2 Suppl 1):S54–62.
Article37. Manoussakis MN, Kapsogeorgou EK. The role of intrinsic epithelial activation in the pathogenesis of Sjögren's syndrome. J Autoimmun. 2010; 35:219–24.
Article38. Reutershan J. CXCR2–the receptor to hit? Drug News Perspect. 2006; 19:615–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Classification Criteria for Primary Sjögren's Syndrome and Salivary Gland Ultrasonography
- A Case of Treatment with Steroid and Hydrochloroquine of Thrombocytopenia in Primary Sjögren's Syndrome
- A Case of Sjögren-Larsson Syndrome
- Ocular Manifestations of Sjögren Syndrome
- Longitudinal Changes of the European League Against Rheumatism Sjögren's Syndrome Patient Reported Index in Korean Patients with Primary Sjögren's Syndrome