Cancer Res Treat.
2016 Oct;48(4):1420-1428. 10.4143/crt.2015.309.
Clinical Practices and Outcomes on Chemotherapy-Induced Nausea and Vomiting Management in South Korea: Comparison with Asia-Pacific Data of the Pan Australasian Chemotherapy Induced Emesis Burden of Illness Study
- Affiliations
-
- 1Department of Internal Medicine, Seoul St. Mary's Hospital, Cancer Research Institution, The Catholic University of Korea, Seoul, Korea.
- 2Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 3Department of Internal Medicine, Dong-A University Hospital, Busan, Korea.
- 4Department of Internal Medicine, Severance Hospital, Yonsei University, Seoul, Korea.
- 5Merck Sharp and Dohme, Subsidiary of Merck and Co., Inc., Seoul, Korea.
- 6Department of Oncology/Hematology, Kyungpook National University Medical Center, Daegu, Korea. jkk21c@knu.ac.kr
- KMID: 2356244
- DOI: http://doi.org/10.4143/crt.2015.309
Abstract
- PURPOSE
This study reported patient outcomes of chemotherapy-induced nausea and vomiting (CINV) prophylaxis for highly emetogenic chemotherapy (HEC) and moderately emetogenic chemotherapy (MEC) regimens and evaluated its adherence to acute-phase CINV prophylaxis in the Korean population subset of the Pan Australasian Chemotherapy Induced Emesis burden of illness (PrACTICE) study.
MATERIALS AND METHODS
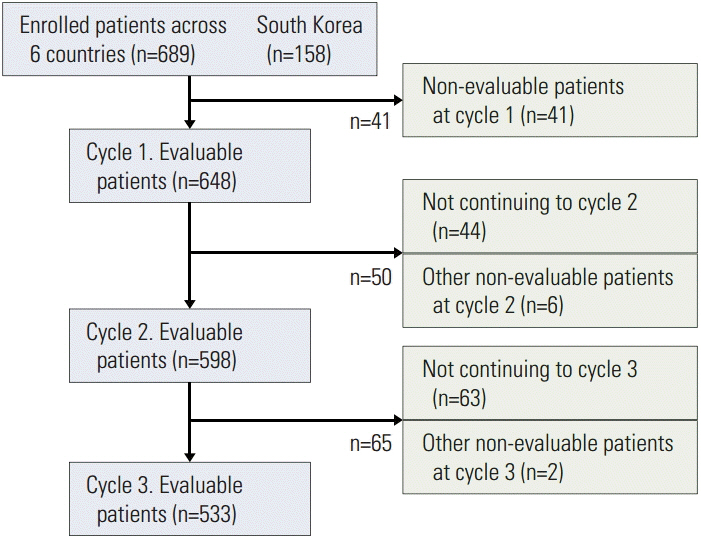
This subgroup analysis evaluated 158 Korean patients receiving HEC or MEC and compared the data (wherever possible) with that of 648 patients from the Asia-Pacific (AP) region. Study endpoints included evaluation of primary CINV prophylaxis and adherence to acute-phase CINV prophylaxis in cycle 1 (American Society of Clinical Oncology [ASCO] Quality Oncology Practice Initiative [QOPI]).
RESULTS
In South Korea and the AP, a 5-hydroxytryptamine-3 receptor antagonist (5HT₃-RA) prophylaxis for the acute phase was administered to 79/80 patients (98.8%) for HEC and 70/71 patients (98.6%) for MEC regimens (QOPI-1). Triple regimen (corticosteroid-5HT₃-RA-neurokinin 1-RA) was initiated in 46/80 patients (57.5%) for prophylaxis of acute CINV in cycle 1 of HEC (QOPI-3). Double regimen (corticosteroid-5HT₃-RA, with or within NKâ‚-RA) was initiated in 61/71 patients (83.1%) for control of acute CINV in cycle 1 of MEC a(QOPI-2).
CONCLUSION
Active management of CINV is necessary in cycle 1 of HEC in South Korea, despite higher rates than the AP region. Adherence to the international guidelines for CINV prophylaxis requires attention in the acute phase in cycle 1 of the HEC regimen.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Jordan K, Gralla R, Jahn F, Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014; 722:197–202.
Article2. Bloechl-Daum B, Deuson RR, Mavros P, Hansen M, Herrstedt J. Delayed nausea and vomiting continue to reduce patients' quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006; 24:4472–8.
Article3. Hassan BA, Yusoff ZB. Genetic polymorphisms in the three malaysian races effect granisetron clinical antiemetic actions in breast cancer patients receiving chemotherapy. Asian Pac J Cancer Prev. 2011; 12:185–91.4. Molassiotis A, Aapro M, Dicato M, Gascon P, Novoa SA, Isambert N, et al. Evaluation of risk factors predicting chemotherapy-related nausea and vomiting: results from a European prospective observational study. J Pain Symptom Manage. 2014; 47:839–48. e4.
Article5. Pirri C, Katris P, Trotter J, Bayliss E, Bennett R, Drummond P. Risk factors at pretreatment predicting treatment-induced nausea and vomiting in Australian cancer patients: a prospective, longitudinal, observational study. Support Care Cancer. 2011; 19:1549–63.
Article6. Basch E, Hesketh PJ, Kris MG, Prestrud AA, Temin S, Lyman GH. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Oncol Pract. 2011; 7:395–8.
Article7. National Comprehensive Cancer Network. Antiemesis [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014. [cited 2014 Dec 26]. Available from: http://www.nccn.org.8. Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010; 21 Suppl 5:v232–43.
Article9. Herrstedt J, Roila F; ESMO Guidelines Working Group. Chemotherapy-induced nausea and vomiting: ESMO clinical recommendations for prophylaxis. Ann Oncol. 2008; 19 Suppl 2:ii110–2.
Article10. Caracuel F, Munoz N, Banos U, Ramirez G. Adherence to antiemetic guidelines and control of chemotherapy-induced nausea and vomiting (CINV) in a large hospital. J Oncol Pharm Pract. 2015; 21:163–9.
Article11. Affronti ML, Schneider SM, Herndon JE 2nd, Schlundt S, Friedman HS. Adherence to antiemetic guidelines in patients with malignant glioma: a quality improvement project to translate evidence into practice. Support Care Cancer. 2014; 22:1897–905.
Article12. Fujii H, Iihara H, Ishihara M, Takahashi T, Yoshida K, Itoh Y. Improvement of adherence to guidelines for antiemetic medication enhances emetic control in patients with colorectal cancer receiving chemotherapy of moderate emetic risk. Anticancer Res. 2013; 33:5549–56.13. Gilmore JW, Peacock NW, Gu A, Szabo S, Rammage M, Sharpe J, et al. Antiemetic guideline consistency and incidence of chemotherapy-induced nausea and vomiting in US community oncology practice: INSPIRE Study. J Oncol Pract. 2014; 10:68–74.
Article14. Hsieh RK, Chan A, Kim HK, Yu S, Kim JG, Lee MA, et al. Baseline patient characteristics, incidence of CINV, and physician perception of CINV incidence following moderately and highly emetogenic chemotherapy in Asia Pacific countries. Support Care Cancer. 2015; 23:263–72.
Article15. Yu S, Burke TA, Chan A, Kim HK, Hsieh RK, Hu X, et al. Antiemetic therapy in Asia Pacific countries for patients receiving moderately and highly emetogenic chemotherapy: a descriptive analysis of practice patterns, antiemetic quality of care, and use of antiemetic guidelines. Support Care Cancer. 2015; 23:273–82.16. Kim HK, Hsieh R, Chan A, Yu S, Han B, Gao Y, et al. Impact of CINV in earlier cycles on CINV and chemotherapy regimen modification in subsequent cycles in Asia Pacific clinical practice. Support Care Cancer. 2015; 23:293–300.
Article17. Chan A, Kim HK, Hsieh RK, Yu S, de Lima Lopes G Jr, Su WC, et al. Incidence and predictors of anticipatory nausea and vomiting in Asia Pacific clinical practice: a longitudinal analysis. Support Care Cancer. 2015; 23:283–91.18. Keefe DM, Chan A, Kim HK, Hsieh RK, Yu S, Wang Y, et al. Rationale and design of the Pan Australasian chemotherapy-induced emesis burden of illness study. Support Care Cancer. 2015; 23:253–61.
Article19. Liau CT, Chu NM, Liu HE, Deuson R, Lien J, Chen JS. Incidence of chemotherapy-induced nausea and vomiting in Taiwan: physicians' and nurses' estimation vs. patients' reported outcomes. Support Care Cancer. 2005; 13:277–86.
Article20. Chan A, Shih V, Chew L. Evolving roles of oncology pharmacists in Singapore: a survey on prescribing patterns of antiemetics for chemotherapy induced nausea and vomiting (CINV) at a cancer centre. J Oncol Pharm Pract. 2008; 14:23–9.
Article21. So WK, Chan DN, Chan HY, Krishnasamy M, Chan T, Ling WM, et al. Knowledge and practice among Hong Kong oncology nurses in the management of chemotherapy-induced nausea and vomiting. Eur J Oncol Nurs. 2013; 17:370–4.
Article22. Hesketh PJ, Kris MG, Grunberg SM, Beck T, Hainsworth JD, Harker G, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997; 15:103–9.
Article23. Molassiotis A, Coventry PA, Stricker CT, Clements C, Eaby B, Velders L, et al. Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage. 2007; 34:148–59.
Article24. Uppsala Monitoring Center. 2010 WHO Drug Dictionary version 3 [Internet]. Uppsala: Uppsala Monitoring Center;2010. [cited 2014 Dec 26]. Available from: http://www.umc-products.com.25. American Society of Clinical Oncology. The Quality Oncology Practice Initiative (QOPI) and the QOPI Certification Program [Internet]. Alexandria, VA: American Society of Clinical Oncology;2014. [cited 2014 Dec 26]. Available from: http://qopi.asco.org/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Granisetron Plus Dexamethasone in the Prevention of Delayed Nausea and Vomiting
- Management of Chemotherapy Induced Nausea and Vomiting
- Factors of Anticipatory Nausea and Vomiting in Cancer Patients
- Comparison of Effects of Different Acupressure Methods on Nausea, Vomiting, and Anorexia for Breast Cancer Patients: Among Patients Undergoing Chemotherapy
- A Comparison of the Acute Antiemetic Effect of Tropisetron with Ondansetron in Patients Receiving Cisplatin