Cancer Res Treat.
2016 Oct;48(4):1177-1186. 10.4143/crt.2015.401.
East Asian Subgroup Analysis of a Randomized, Double-Blind, Phase 3 Study of Docetaxel and Ramucirumab Versus Docetaxel and Placebo in the Treatment of Stage IV Non-small Cell Lung Cancer Following Disease Progression after One Prior Platinum-Based Therapy (REVEL)
- Affiliations
-
- 1Division of Hematology-Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 3Division of Hematology, Department of Internal Medicine, Gachon University Gil Hospital, Incheon, Korea.
- 4Department of Medical Oncology, College of Medicine, The Catholic University of Korea, St. Mary's Hospital, Seoul, Korea.
- 5Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan.
- 6Eli Lilly and Company, Indianapolis, IN, USA.
- 7Eli Lilly and Company, Gurgaon, Haryana, India.
- 8Eli Lilly Interamerica, Buenos Aires, Argentina. orlando_mauro@lilly.com
- KMID: 2356220
- DOI: http://doi.org/10.4143/crt.2015.401
Abstract
- PURPOSE
REVEL demonstrated improved overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) with docetaxel+ramucirumab versus docetaxel+placebo in 1,253 intent-to-treat (ITT) stage IV non-small cell lung cancer patients with disease progression following platinum-based chemotherapy. Results from the East Asian subgroup analysis are reported.
MATERIALS AND METHODS
Subgroup analyses were performed in the East Asian ITT population (n=89). Kaplan-Meier analysis and Cox proportional hazards regression were performed for OS and PFS, and the Cochran-Mantel-Haenszel test was performed for response rate.
RESULTS
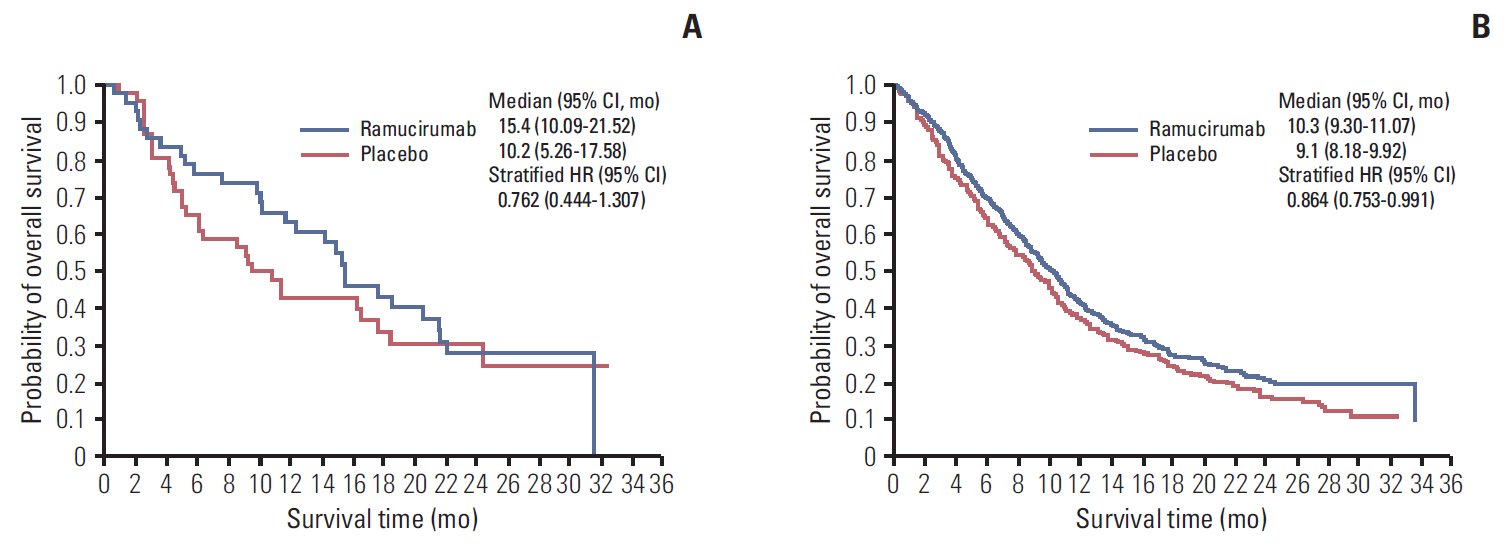
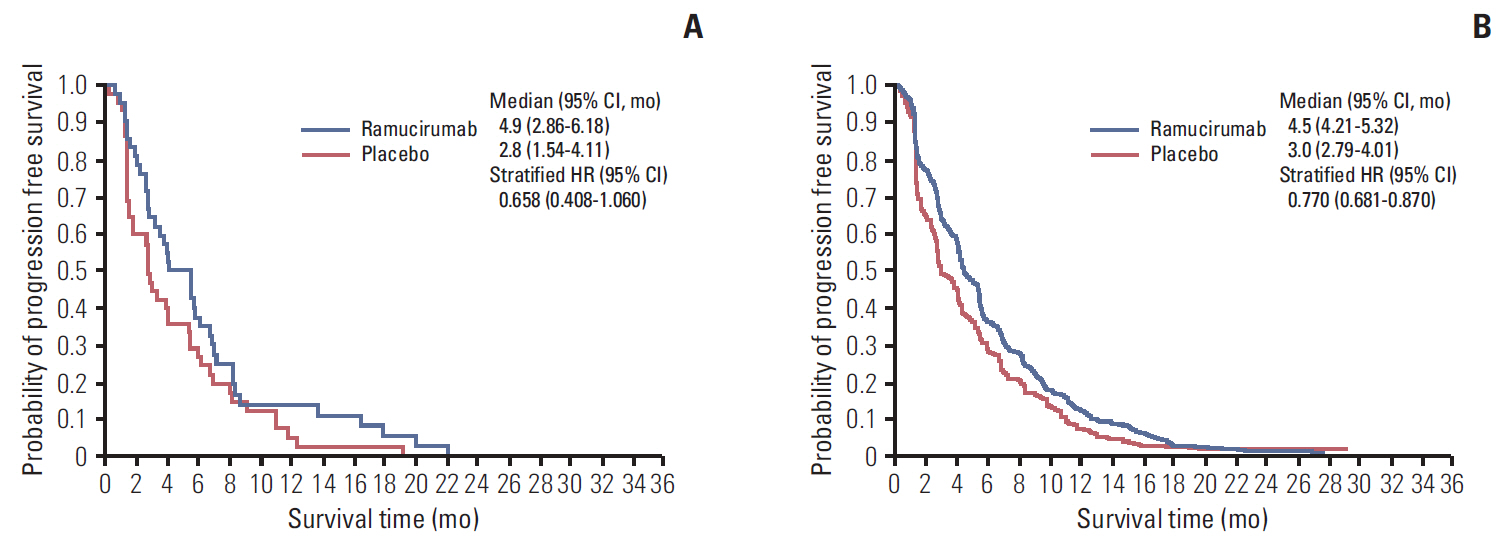
In docetaxel+ramucirumab (n=43) versus docetaxel+placebo (n=46), median OS was 15.44 months versus 10.17 months (hazard ratio [HR], 0.762; 95% confidence interval [CI], 0.444 to 1.307), median PFS was 4.88 months versus 2.79 months (HR, 0.658; 95% CI, 0.408 to 1.060), and ORR was 25.6% (95% CI, 13.5 to 41.2) versus 8.7% (95% CI, 2.4 to 20.8). Due to increased incidence of neutropenia and febrile neutropenia in East Asian patients, starting dose of docetaxel was reduced for newly enrolled East Asian patients (75 to 60 mg/m², n=24). In docetaxel+ramucirumab versus docetaxel+placebo, incidence of neutropenia was 84.4% versus 72.7% (75 mg/m²) and 54.5% versus 38.5% (60 mg/m²). Incidence of febrile neutropenia was 43.8% versus 12.1% (75 mg/m²) and 0% versus 7.7% (60 mg/m²).
CONCLUSION
Results of this subgroup analysis showed a trend favoring ramucirumab+docetaxel for median OS, PFS, and improved ORR in East Asian patients, consistent with ITT population results. Reduction of starting dose of docetaxel in East Asian patients was associated with improved safety.
MeSH Terms
Figure
Cited by 1 articles
-
Crizotinib versus Chemotherapy in Asian Patients with
ALK -Positive Advanced Non-small Cell Lung Cancer
Makoto Nishio, Dong-Wan Kim, Yi-Long Wu, Kazuhiko Nakagawa, Benjamin J. Solomon, Alice T. Shaw, Satoshi Hashigaki, Emiko Ohki, Tiziana Usari, Jolanda Paolini, Anna Polli, Keith D. Wilner, Tony Mok
Cancer Res Treat. 2018;50(3):691-700. doi: 10.4143/crt.2017.280.
Reference
-
References
1. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer;2013. [cited 2014 Jan 27]. Available from: http://globocan.iarc.fr.2. Schnabel PA, Smit E, Carpeno JC, Lesniewski-Kmak K, Aerts J, Kraaij K, et al. Influence of histology and biomarkers on first-line treatment of advanced non-small cell lung cancer in routine care setting: baseline results of an observational study (FRAME). Lung Cancer. 2012; 78:263–9.
Article3. Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol. 2012; 19(Suppl 1):S52–8.
Article4. Gerber DE, Schiller JH. Maintenance chemotherapy for advanced non-small-cell lung cancer: new life for an old idea. J Clin Oncol. 2013; 31:1009–20.
Article5. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–103.
Article6. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005; 353:123–32.
Article7. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, von Pawel J, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004; 22:1589–97.
Article8. Jantus-Lewintre E, Sanmartin E, Sirera R, Blasco A, Sanchez JJ, Taron M, et al. Combined VEGF-A and VEGFR-2 concentrations in plasma: diagnostic and prognostic implications in patients with advanced NSCLC. Lung Cancer. 2011; 74:326–31.
Article9. Tugues S, Koch S, Gualandi L, Li X, Claesson-Welsh L. Vascular endothelial growth factors and receptors: anti-angiogenic therapy in the treatment of cancer. Mol Aspects Med. 2011; 32:88–111.
Article10. Skobe M, Rockwell P, Goldstein N, Vosseler S, Fusenig NE. Halting angiogenesis suppresses carcinoma cell invasion. Nat Med. 1997; 3:1222–7.
Article11. Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006; 355:2542–50.
Article12. Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol. 2010; 21:1804–9.
Article13. Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010; 28:780–7.14. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.
Article15. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–9.
Article16. Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015; 16:499–508.
Article17. Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007; 2:430–9.
Article18. Ou SH, Ziogas A, Zell JA. Asian ethnicity is a favorable prognostic factor for overall survival in non-small cell lung cancer (NSCLC) and is independent of smoking status. J Thorac Oncol. 2009; 4:1083–93.
Article19. Hasegawa Y, Kawaguchi T, Kubo A, Ando M, Shiraishi J, Isa S, et al. Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol. 2011; 6:1881–8.
Article20. Garon EB, Ciuleanu TE, Arrieta O, Prabhash K, Syrigos KN, Goksel T, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014; 384:665–73.
Article21. Goh BC, Lee SC, Wang LZ, Fan L, Guo JY, Lamba J, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002; 20:3683–90.
Article22. Goh BC, Lehnert M, Lim HL, Ng AW, Chan CC, Kong HL, et al. Phase II trial of docetaxel in Asian patients with inoperable stage III non-small cell lung cancer. Acta Oncol. 2000; 39:225–9.23. Kenmotsu H, Tanigawara Y. Pharmacokinetics, dynamics and toxicity of docetaxel: why the Japanese dose differs from the Western dose. Cancer Sci. 2015; 106:497–504.
Article24. Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE, et al. Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res. 2010; 16:3548–61.
Article25. Crino L, Metro G. Therapeutic options targeting angiogenesis in nonsmall cell lung cancer. Eur Respir Rev. 2014; 23:79–91.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Death Induction Mechanism of Non-small Cell Lung Cancer Cell Line, NCI-H1703 by Docetaxel
- Docetaxel as Second-line Monotherapy for Advanced Non-small Cell Lung Cancer
- A Phase II Trial of Docetaxel and Ifosfamide for Patients with Platinum-Resistant or Refractory Non-Small Cell Lung Cancer in a Salvage Setting
- Comparison of Gefitinib versus Docetaxel in Patients with Pre-Treated Non-Small Cell Lung Cancer (NSCLC)
- Treatment of Advanced and Metastatic Squamous Non-small Cell Lung Cancer