Personalized Medicine in Allergy
- Affiliations
-
- 1Allergy & Respiratory Diseases, DIMI Department of Internal Medicine, IRCCS AOU San Martino-IST, University of Genoa, Genoa, Italy. canonica@unige.it
- 2Division of Clinical Immunology and Allergy, Department of Translational Medical Sciences, University of Naples Federico II, Naples, Italy.
- KMID: 2355890
- DOI: http://doi.org/10.4168/aair.2017.9.1.15
Abstract
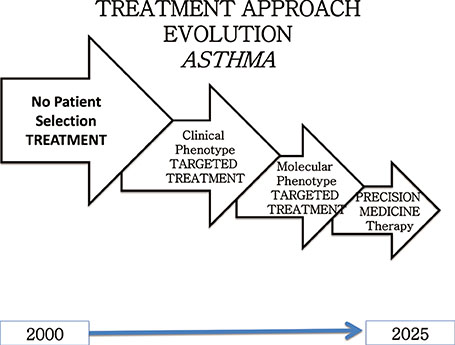
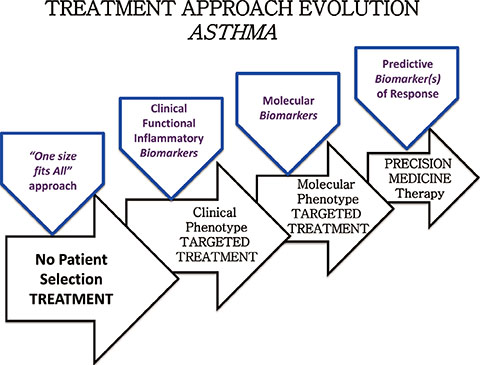
- Allergic disease is among the most common pathologies worldwide and its prevalence has constantly increased up to the present days, even if according to the most recent data it seems to be slightly slowing down. Allergic disease has not only a high rate of misdiagnosis and therapeutic inefficacy, but represents an enormous, resource-absorbing black hole in respiratory and general medicine. The aim of this paper is to summarize principal therapeutic innovations in atopic disease management befallen in the recent years in terms of personalized/precision medicine.
MeSH Terms
Figure
Cited by 5 articles
-
Serum Periostin Is Negatively Correlated With Exposure to Formaldehyde and Volatile Organic Compounds in Children
Dong Keon Yon, Jaewoo An, Eun Kyo Ha, Hye Mi Jee, Kenji Izuhara, Junya Ono, Young-Ho Jung, Kyung Suk Lee, Youn Ho Sheen, Heysung Baek, Man Yong Han
Allergy Asthma Immunol Res. 2018;10(6):716-721. doi: 10.4168/aair.2018.10.6.716.Altered Sphingolipid Metabolism Is Associated With Asthma Phenotype in House Dust Mite-Allergic Patients
Krzysztof Kowal, Ewa Żebrowska, Adrian Chabowski
Allergy Asthma Immunol Res. 2019;11(3):330-342. doi: 10.4168/aair.2019.11.3.330.Serum Periostin Levels: A Potential Serologic Marker for Toluene Diisocyanate-Induced Occupational Asthma
Ji-Ho Lee, Sang-Ha Kim, Youngwoo Choi, Hoang Kim Tu Trinh, Eun-Mi Yang, Ga-Young Ban, Yoo Seob Shin, Young-Min Ye, Kenji Izuhara, Hae-Sim Park
Yonsei Med J. 2018;59(10):1214-1221. doi: 10.3349/ymj.2018.59.10.1214.Perceptions of Severe Asthma and Asthma-COPD Overlap Syndrome Among Specialists: A Questionnaire Survey
Sang-Heon Kim, Ji Yong Moon, Jae Hyun Lee, Ga-Young Ban, Sujeong Kim, Mi-Ae Kim, Joo-Hee Kim, Min-Hye Kim, Chan-Sun Park, So-Young Park, Hyouk-Soo Kwon, Jae-Woo Kwon, Jae-Woo Jung, Hye-Ryun Kang, Jong-Sook Park, Tae-Bum Kim, Heung-Woo Park, You Sook Cho, Kwang-Ha Yoo, Yeon-Mok Oh, Byung-Jae Lee, An-Soo Jang, Sang-Heon Cho, Hae-Sim Park, Choon-Sik Park, Ho Joo Yoon,
Allergy Asthma Immunol Res. 2018;10(3):225-235. doi: 10.4168/aair.2018.10.3.225.Tolerogenic Dendritic Cells Reduce Airway Inflammation in a Model of Dust Mite Triggered Allergic Inflammation
Luciana S. Aragão-França, Viviane C. J. Rocha, Andre Cronemberger-Andrade, F. H. B. Costa, José Fernandes Vasconcelos, Daniel Abensur Athanazio, Daniela Nascimento Silva, E. S. Santos, Cássio Santana Meira, C. F. Araújo, Jéssica Vieira Cerqueira, Fabíola Cardillo, Neuza Maria Alcântara-Neves, Milena Botelho Pereira Soares, Lain C. Pontes-de-Carvalho
Allergy Asthma Immunol Res. 2018;10(4):406-419. doi: 10.4168/aair.2018.10.4.406.
Reference
-
1. Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015; 135:299–310.2. Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006; 368:733–743.3. Condizioni di salute, fattori di rischio e ricorso ai servizi sanitari: anno 2005 [Internet]. Rome: Instituto nazionale di statistica;2007. cited 2016 Jan 13. Available from: http://www3.istat.it/salastampa/comunicati/non_calendario/20070302_00/testointegrale.pdf.4. Accordini S, Corsico AG, Braggion M, Gerbase MW, Gislason D, Gulsvik A, et al. The cost of persistent asthma in Europe: an international population-based study in adults. Int Arch Allergy Immunol. 2013; 160:93–101.5. Pawankar R, Canonica GW, Holgate ST, Lockey RF, editors. WAO White book on allergy [Internet]. Milwaukee (WI): World Allergy Organization;2011. cited 2016 Jan 13. Available from: http://www.worldallergy.org/UserFiles/file/WAO-White-Book-on-Allergy_web.pdf.6. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Summary health statistics for U.S. children: national health interview survey, 2012. Hyattsville (MD): U.S. Department of Health and Human Services;2013.7. Saini S, Rosen KE, Hsieh HJ, Wong DA, Conner E, Kaplan A, et al. A randomized, placebo-controlled, dose-ranging study of single-dose omalizumab in patients with H1-antihistamine-refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011; 128:567–573.e1.8. Kaplan A, Ledford D, Ashby M, Canvin J, Zazzali JL, Conner E, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013; 132:101–109.9. Darveaux J, Busse WW. Biologics in asthma--the next step toward personalized treatment. J Allergy Clin Immunol Pract. 2015; 3:152–160.10. Ferrando M, Bagnasco D, Braido F, Varricchi G, Canonica GW. Biosimilars in allergic diseases. Curr Opin Allergy Clin Immunol. 2016; 16:68–73.11. Tarantini F, Baiardini I, Passalacqua G, Braido F, Canonica GW. Asthma treatment: 'magic bullets which seek their own targets'. Allergy. 2007; 62:605–610.12. Schwartz RS. Paul Ehrlich's magic bullets. N Engl J Med. 2004; 350:1079–1080.13. Passalacqua G, Canonica GW. AIT (allergen immunotherapy): a model for the "precision medicine". Clin Mol Allergy. 2015; 13:24.14. Canonica GW, Bachert C, Hellings P, Ryan D, Valovirta E, Wickman M, et al. Allergen Immunotherapy (AIT): a prototype of precision medicine. World Allergy Organ J. 2015; 8:31.15. Bjermer L. Time for a paradigm shift in asthma treatment: from relieving bronchospasm to controlling systemic inflammation. J Allergy Clin Immunol. 2007; 120:1269–1275.16. Baird B, Shopes RJ, Oi VT, Erickson J, Kane P, Holowka D. Interaction of IgE with its high-affinity receptor. Structural basis and requirements for effective cross-linking. Int Arch Allergy Appl Immunol. 1989; 88:23–28.17. Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci USA. 2014; 111:367–372.18. Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, et al. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001; 108:184–190.19. Sorkness CA, Wildfire JJ, Calatroni A, Mitchell HE, Busse WW, O'Connor GT, et al. Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunol Pract. 2013; 1:163–171.20. Humbert M, Beasley R, Ayres J, Slavin R, Hébert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005; 60:309–316.21. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011; 364:1005–1015.22. Rodrigo GJ, Neffen H, Castro-Rodriguez JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011; 139:28–35.23. Global Initiative for Asthma [Internet]. cited 2016 Jan 20. Available from: www.ginasthma.org.24. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811.25. Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014; 44:1371–1385.26. Gauvreau GM, Harris JM, Boulet LP, Scheerens H, Fitzgerald JM, Putnam WS, et al. Targeting membrane-expressed IgE B cell receptor with an antibody to the M1 prime epitope reduces IgE production. Sci Transl Med. 2014; 6:243ra85.27. O'Byrne PM, Inman MD, Parameswaran K. The trials and tribulations of IL-5, eosinophils, and allergic asthma. J Allergy Clin Immunol. 2001; 108:503–508.28. Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014; 371:1189–1197.29. Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012; 380:651–659.30. Kim S, Marigowda G, Oren E, Israel E, Wechsler ME. Mepolizumab as a steroid-sparing treatment option in patients with Churg-Strauss syndrome. J Allergy Clin Immunol. 2010; 125:1336–1343.31. Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008; 358:1215–1228.32. Gevaert P, Van Bruaene N, Cattaert T, Van Steen K, Van Zele T, Acke F, et al. Mepolizumab, a humanized anti-IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011; 128:989–995.e1-8.33. Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK, et al. Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2006; 118:1312–1319.34. Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005; 60:693–696.35. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015; 3:355–366.36. Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014; 2:879–890.37. Kolbeck R, Kozhich A, Koike M, Peng L, Andersson CK, Damschroder MM, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010; 125:1344–1353.e2.38. Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G 3rd, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2012; 129:456–463. 463.e1–463.e3.39. Gevaert P, Lang-Loidolt D, Lackner A, Stammberger H, Staudinger H, Van Zele T, et al. Nasal IL-5 levels determine the response to anti-IL-5 treatment in patients with nasal polyps. J Allergy Clin Immunol. 2006; 118:1133–1141.40. Brightling CE, Bleecker ER, Panettieri RA Jr, Bafadhel M, She D, Ward CK, et al. Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med. 2014; 2:891–901.41. Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW. IL-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs. Curr Opin Allergy Clin Immunol. 2016; 16:186–200.42. Lötvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011; 127:355–360.43. De Ferrari L, Chiappori A, Bagnasco D, Riccio AM, Passalacqua G, Canonica GW. Molecular phenotyping and biomarker development: are we on our way towards targeted therapy for severe asthma? Expert Rev Respir Med. 2016; 10:29–38.44. Chiappori A, De Ferrari L, Folli C, Mauri P, Riccio AM, Canonica GW. Biomarkers and severe asthma: a critical appraisal. Clin Mol Allergy. 2015; 13:20.45. van den Toorn LM, Overbeek SE, de Jongste JC, Leman K, Hoogsteden HC, Prins JB. Airway inflammation is present during clinical remission of atopic asthma. Am J Respir Crit Care Med. 2001; 164:2107–2113.46. Sippel JM, Holden WE, Tilles SA, O'Hollaren M, Cook J, Thukkani N, et al. Exhaled nitric oxide levels correlate with measures of disease control in asthma. J Allergy Clin Immunol. 2000; 106:645–650.47. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011; 184:602–615.48. Ricciardolo FL, Sorbello V, Ciprandi G. FeNO as biomarker for asthma phenotyping and management. Allergy Asthma Proc. 2015; 36:e1–e8.49. Horváth I, Hunt J, Barnes PJ, Alving K, Antczak A, Baraldi E, et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005; 26:523–548.50. Murugan A, Prys-Picard C, Calhoun WJ. Biomarkers in asthma. Curr Opin Pulm Med. 2009; 15:12–18.51. Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010; 107:14170–14175.52. Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012; 122:2590–2600.53. Corren J, Lemanske RF Jr, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011; 365:1088–1098.54. Wu D, Zhou J, Bi H, Li L, Gao W, Huang M, et al. CCL11 as a potential diagnostic marker for asthma? J Asthma. 2014; 51:847–854.55. Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Respir Med. 2010; 104:188–196.56. Katz LE, Gleich GJ, Hartley BF, Yancey SW, Ortega HG. Blood eosinophil count is a useful biomarker to identify patients with severe eosinophilic asthma. Ann Am Thorac Soc. 2014; 11:531–536.57. Cicardi M, Aberer W, Banerji A, Bas M, Bernstein JA, Bork K, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy. 2014; 69:602–616.58. Lin RY, Cannon AG, Teitel AD. Pattern of hospitalizations for angioedema in New York between 1990 and 2003. Ann Allergy Asthma Immunol. 2005; 95:159–166.59. Bas M, Greve J, Strassen U, Khosravani F, Hoffmann TK, Kojda G. Angioedema induced by cardiovascular drugs: new players join old friends. Allergy. 2015; 70:1196–1200.60. Cicardi M, Agostoni A. Hereditary angioedema. N Engl J Med. 1996; 334:1666–1667.61. Longhurst H, Cicardi M. Hereditary angio-oedema. Lancet. 2012; 379:474–481.62. Loffredo S, Bova M, Suffritti C, Borriello F, Zanichelli A, Petraroli A, et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy. Forthcoming. 2016.63. Donaldson VH, Rosen FS. Action of complement in hereditary angioneurotic edema: the role of c'1-esterase. J Clin Invest. 1964; 43:2204–2213.64. Nagy N, Grattan CE, McGrath JA. New insights into hereditary angio-oedema: Molecular diagnosis and therapy. Australas J Dermatol. 2010; 51:157–162.65. Bafunno V, Bova M, Loffredo S, Divella C, Petraroli A, Marone G, et al. Mutational spectrum of the c1 inhibitor gene in a cohort of Italian patients with hereditary angioedema: description of nine novel mutations. Ann Hum Genet. 2014; 78:73–82.66. Pappalardo E, Cicardi M, Duponchel C, Carugati A, Choquet S, Agostoni A, et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol. 2000; 106:1147–1154.67. Bygum A, Fagerberg CR, Ponard D, Monnier N, Lunardi J, Drouet C. Mutational spectrum and phenotypes in Danish families with hereditary angioedema because of C1 inhibitor deficiency. Allergy. 2011; 66:76–84.68. Zanichelli A, Magerl M, Longhurst H, Fabien V, Maurer M. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol. 2013; 9:29.69. Caballero T, Aygören-Pürsün E, Bygum A, Beusterien K, Hautamaki E, Sisic Z, et al. The humanistic burden of hereditary angioedema: results from the Burden of Illness Study in Europe. Allergy Asthma Proc. 2014; 35:47–53.70. Betschel S, Badiou J, Binkley K, Hébert J, Kanani A, Keith P, et al. Canadian hereditary angioedema guideline. Allergy Asthma Clin Immunol. 2014; 10:50.71. Li HH, Moldovan D, Bernstein JA, Reshef A, Porebski G, Stobiecki M, et al. Recombinant human-C1 inhibitor is effective and safe for repeat hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2015; 3:417–423.72. Longhurst HJ, Aberer W, Bouillet L, Caballero T, Fabien V, Zanichelli A, et al. Analysis of characteristics associated with reinjection of icatibant: results from the icatibant outcome survey. Allergy Asthma Proc. 2015; 36:399–406.73. Petraroli A, Squeglia V, Di Paola N, Barbarino A, Bova M, Spanò R, et al. Home therapy with plasma-derived C1 inhibitor: a strategy to improve clinical outcomes and costs in hereditary angioedema. Int Arch Allergy Immunol. 2015; 166:259–266.74. Cicardi M, Levy RJ, McNeil DL, Li HH, Sheffer AL, Campion M, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med. 2010; 363:523–531.75. Riedl MA. Critical appraisal of androgen use in hereditary angioedema: a systematic review. Ann Allergy Asthma Immunol. 2015; 114:281–288.e7.76. Freda MF, Savarese L, Bova M, De Falco R, De Luca Picione R, Galante A, et al. Stress and psychological factors in the variable clinical phenotype of hereditary angioedema: a pilot study. Pediatr Allergy Immunol Pulmonol. 2016; 29:6–12.77. Cooke A, Bulkhi A, Casale TB. Role of biologics in intractable urticaria. Biologics. 2015; 9:25–33.78. Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015; 135:337–342.e2.79. Maurer M, Rosén K, Hsieh HJ, Saini S, Grattan C, Gimenéz-Arnau A, et al. Omalizumab for the treatment of chronic idiopathic or spontaneous urticaria. N Engl J Med. 2013; 368:924–935.80. Strunk RC, Bloomberg GR. Omalizumab for asthma. N Engl J Med. 2006; 354:2689–2695.81. Detoraki A, Di Capua L, Varricchi G, Genovese A, Marone G, Spadaro G. Omalizumab in patients with eosinophilic granulomatosis with polyangiitis: a 36-month follow-up study. J Asthma. 2016; 53:201–206.82. Menzella F, Piro R, Facciolongo N, Castagnetti C, Simonazzi A, Zucchi L. Long-term benefits of omalizumab in a patient with severe non-allergic asthma. Allergy Asthma Clin Immunol. 2011; 7:9.83. de Llano LP, Vennera MC, Álvarez FJ, Medina JF, Borderías L, Pellicer C, et al. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. J Asthma. 2013; 50:296–301.84. Lavaud F, Bonniaud P, Dalphin JC, Leroyer C, Muller D, Tannous R, et al. Usefulness of omalizumab in ten patients with severe occupational asthma. Allergy. 2013; 68:813–815.85. Tanou K, Zintzaras E, Kaditis AG. Omalizumab therapy for allergic bronchopulmonary aspergillosis in children with cystic fibrosis: a synthesis of published evidence. Pediatr Pulmonol. 2014; 49:503–507.86. Jat KR, Walia DK, Khairwa A. Anti-IgE therapy for allergic bronchopulmonary aspergillosis in people with cystic fibrosis. Cochrane Database Syst Rev. 2015; 11:CD010288.87. Hotze M, Baurecht H, Rodríguez E, Chapman-Rothe N, Ollert M, Fölster-Holst R, et al. Increased efficacy of omalizumab in atopic dermatitis patients with wild-type filaggrin status and higher serum levels of phosphatidylcholines. Allergy. 2014; 69:132–135.88. Yu KK, Crew AB, Messingham KA, Fairley JA, Woodley DT. Omalizumab therapy for bullous pemphigoid. J Am Acad Dermatol. 2014; 71:468–474.89. Kuehr J, Brauburger J, Zielen S, Schauer U, Kamin W, Von Berg A, et al. Efficacy of combination treatment with anti-IgE plus specific immunotherapy in polysensitized children and adolescents with seasonal allergic rhinitis. J Allergy Clin Immunol. 2002; 109:274–280.90. Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013; 131:110–116.e1.91. Pinto JM, Mehta N, DiTineo M, Wang J, Baroody FM, Naclerio RM. A randomized, double-blind, placebo-controlled trial of anti-IgE for chronic rhinosinusitis. Rhinology. 2010; 48:318–324.92. Kanazawa H, Yoshida N, Iino Y. New insights into eosinophilic otitis media. Curr Allergy Asthma Rep. 2015; 15:76.93. Iino Y, Hara M, Hasegawa M, Matsuzawa S, Shinnabe A, Kanazawa H, et al. Clinical efficacy of anti-IgE therapy for eosinophilic otitis media. Otol Neurotol. 2012; 33:1218–1224.94. Iino Y, Hara M, Hasegawa M, Matsuzawa S, Shinnabe A, Kanazawa H, et al. Effect of omalizumab on biomarkers in middle ear effusion in patients with eosinophilic otitis media. Acta Otolaryngol. 2014; 134:366–372.95. Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010; 125:383–389.96. Bégin P, Dominguez T, Wilson SP, Bacal L, Mehrotra A, Kausch B, et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using omalizumab. Allergy Asthma Clin Immunol. 2014; 10:7.97. Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012; 130:1123–1129.e2.98. Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-Il-13 and Il-4 strategy in severe asthma. Int Arch Allergy Immunol. Forthcoming. 2016.99. Braido F, Corsico A, Rogkakou A, Ronzoni V, Baiardini I, Canonica GW. The relationship between allergen immunotherapy and omalizumab for treating asthma. Expert Rev Respir Med. 2015; 9:129–134.