J Clin Neurol.
2016 Apr;12(2):209-217. 10.3988/jcn.2016.12.2.209.
Dissociation of Structural and Functional Integrities of the Motor System in Amyotrophic Lateral Sclerosis and Behavioral-Variant Frontotemporal Dementia
- Affiliations
-
- 1Department of Neurology, Hallym University College of Medicine, Seoul, Korea.
- 2Neuroscience Research Australia, Sydney, Australia.
- 3School of Medical Sciences, University of New South Wales, Sydney, Australia.
- 4Department of Psychiatry, University of Cambridge, Cambridge, UK.
- 5Department of Neurology, Westmead Clinical School, University of Sydney, Westmead, Australia.
- 6Brain and Mind Institute, University of Sydney, Sydney, Australia.
- 7Norwich Medical School, University of East Anglia, Norwich, UK. m.hornberger@uea.ac.uk
- KMID: 2354142
- DOI: http://doi.org/10.3988/jcn.2016.12.2.209
Abstract
- BACKGROUND AND PURPOSE
This study investigated the structural and functional changes in the motor system in amyotrophic lateral sclerosis (ALS; n=25) and behavioral-variant fronto-temporal dementia (bvFTD; n=17) relative to healthy controls (n=37).
METHODS
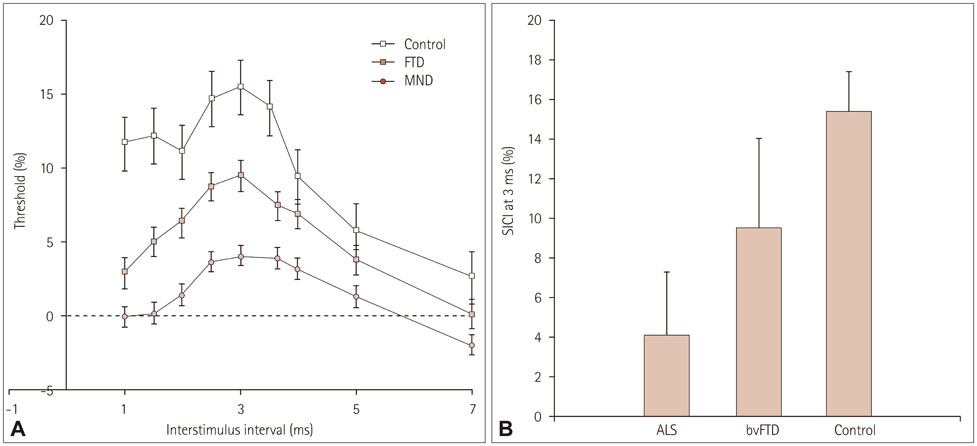
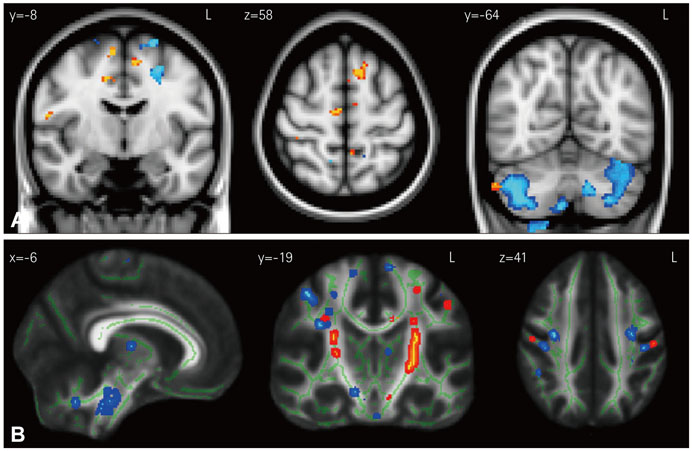
Structural changes were examined using a region-of-interest approach, applying voxel-based morphometry for gray-matter changes and diffusion tensor imaging for white-matter changes. Functional changes in the motor system were elucidated using threshold-tracking transcranial magnetic stimulation (TMS) measurements of upper motor-neuron excitability.
RESULTS
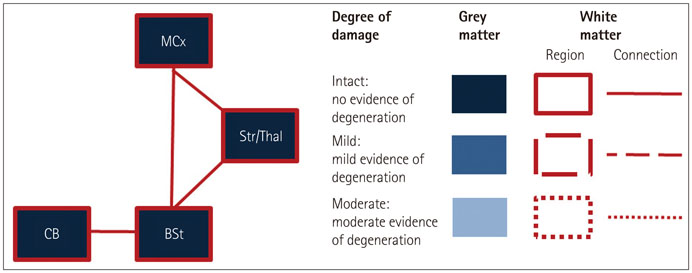
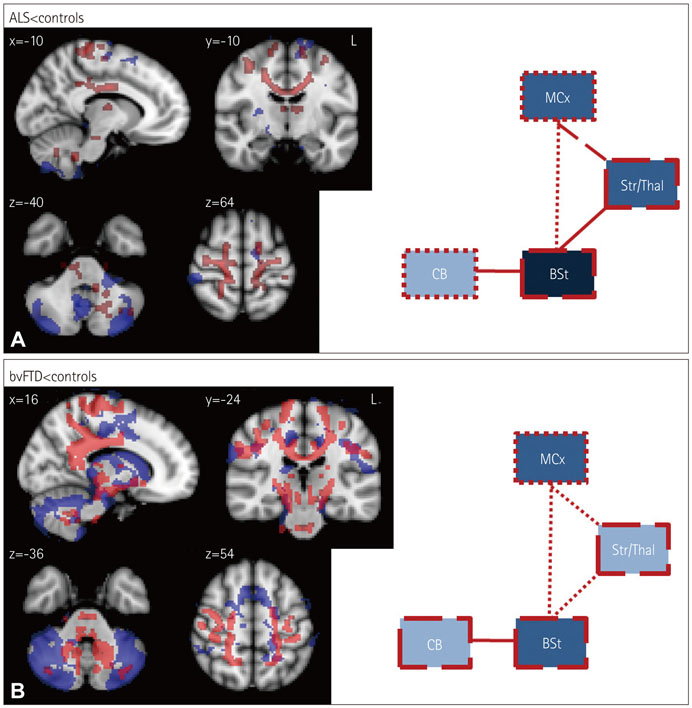
The structural analyses showed that in ALS there were more white-matter changes in the corticospinal and motor-cortex regions and more gray-matter changes in the cerebellum in comparison to controls. bvFTD showed substantial gray- and white-matter changes across virtually all motor-system regions compared to controls, although the brainstem was affected less than the other regions. Direct comparisons across patient groups showed that the gray- and white-matter motor-system changes inclusive of the motor cortex were greater in bvFTD than in ALS. By contrast, the functional integrity of the motor system was more adversely affected in ALS than in bvFTD, with both patient groups showing increased excitability of upper motor neurons compared to controls.
CONCLUSIONS
Cross-correlation of structural and functional data further revealed a neural dissociation of different motor-system regions and tracts covarying with the TMS excitability across both patient groups. The structural and functional motor-system integrities appear to be dissociated between ALS and bvFTD, which represents useful information for the diagnosis of motor-system changes in these two disorders.
Keyword
MeSH Terms
Figure
Reference
-
1. Clark CM, Forman MS. Frontotemporal lobar degeneration with motor neuron disease: a clinical and pathological spectrum. Arch Neurol. 2006; 63:489–490.
Article2. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011; 377:942–955.
Article3. Mackenzie IR, Rademakers R, Neumann M. TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia. Lancet Neurol. 2010; 9:995–1007.
Article4. Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M. Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS One. 2012; 7:e43993.
Article5. Vucic S, Kiernan MC. Novel threshold tracking techniques suggest that cortical hyperexcitability is an early feature of motor neuron disease. Brain. 2006; 129(Pt 9):2436–2446.
Article6. Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008; 131(Pt 6):1540–1550.
Article7. Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011; 134(Pt 9):2582–2594.
Article8. Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011; 134(Pt 9):2456–2477.9. Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–299.
Article10. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999; 169:13–21.
Article11. Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006; 21:1078–1085.
Article12. Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges J, et al. The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry. 2008; 79:500–503.
Article13. Bae JS, Simon NG, Menon P, Vucic S, Kiernan MC. The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. J Clin Neurol. 2013; 9:65–74.
Article14. Vucic S, Ziemann U, Eisen A, Hallett M, Kiernan MC. Transcranial magnetic stimulation and amyotrophic lateral sclerosis: pathophysiological insights. J Neurol Neurosurg Psychiatry. 2013; 84:1161–1170.
Article15. Chow TW, Izenberg A, Binns MA, Freedman M, Stuss DT, Scott CJ, et al. Magnetic resonance imaging in frontotemporal dementia shows subcortical atrophy. Dement Geriatr Cogn Disord. 2008; 26:79–88.
Article16. Garibotto V, Borroni B, Agosti C, Premi E, Alberici A, Eickhoff SB, et al. Subcortical and deep cortical atrophy in frontotemporal lobar degeneration. Neurobiol Aging. 2011; 32:875–884.
Article17. Looi JC, Lindberg O, Zandbelt BB, Ostberg P, Andersen C, Botes L, et al. Caudate nucleus volumes in frontotemporal lobar degeneration: differential atrophy in subtypes. AJNR Am J Neuroradiol. 2008; 29:1537–1543.
Article18. Cardenas VA, Boxer AL, Chao LL, Gorno-Tempini ML, Miller BL, Weiner MW, et al. Deformation-based morphometry reveals brain atrophy in frontotemporal dementia. Arch Neurol. 2007; 64:873–877.
Article19. Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009; 8:94–109.
Article20. Braak H, Ludolph A, Thal DR, Del Tredici K. Amyotrophic lateral sclerosis: dash-like accumulation of phosphorylated TDP-43 in somatodendritic and axonal compartments of somatomotor neurons of the lower brainstem and spinal cord. Acta Neuropathol. 2010; 120:67–74.
Article21. Chen Z, Ma L. Grey matter volume changes over the whole brain in amyotrophic lateral sclerosis: a voxel-wise meta-analysis of voxel based morphometry studies. Amyotroph Lateral Scler. 2010; 11:549–554.
Article22. Mezzapesa DM, Ceccarelli A, Dicuonzo F, Carella A, De Caro MF, Lopez M, et al. Whole-brain and regional brain atrophy in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2007; 28:255–259.23. Mioshi E, Lillo P, Yew B, Hsieh S, Savage S, Hodges JR, et al. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology. 2013; 80:1117–1123.
Article24. Thivard L, Pradat PF, Lehéricy S, Lacomblez L, Dormont D, Chiras J, et al. Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry. 2007; 78:889–892.
Article25. Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012; 135(Pt 3):736–750.
Article26. Sha SJ, Takada LT, Rankin KP, Yokoyama JS, Rutherford NJ, Fong JC, et al. Frontotemporal dementia due to C9ORF72 mutations: clinical and imaging features. Neurology. 2012; 79:1002–1011.
Article27. Keil C, Prell T, Peschel T, Hartung V, Dengler R, Grosskreutz J. Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci. 2012; 13:141.
Article28. Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, et al. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. 2011; 258:647–655.
Article29. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009; 32:413–434.
Article30. Snowden JS, Rollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, et al. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012; 135(Pt 3):693–708.
Article31. Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012; 135(Pt 3):794–806.
Article32. Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006; 63:506–512.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Frontotemporal Dementia with Amyotrophic Lateral Sclerosis Presenting with Pathological Gambling
- Apraxia of Eyelid Closure and Motor Impersistence of Eyelid in a Patient with Amyotrophic Lateral Sclerosis
- Unstable Repeat Expansion in Neurodegenerative Dementias: Mechanisms of Disease
- The Pathogenetic Role of TAR DNA Binding Protein (TDP-43) in Amyotrophic Lateral Sclerosis and Frontotemporal Dementia
- Syndrome of Progressive Bulbar Palsy in Amyotrophic Lateral Sclerosis: A Case Report