Lab Anim Res.
2016 Sep;32(3):160-165. 10.5625/lar.2016.32.3.160.
The effects of dosage and the routes of administrations of streptozotocin and alloxan on induction rate of type1 diabetes mellitus and mortality rate in rats
- Affiliations
-
- 1Department of Biology and Anatomical sciences, School of medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Department of Biology and Anatomical sciences , School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 3Department of Statistics, Qom University, Qom, Iran.
- 4School of medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 5Department of Biology and Anatomical sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran. mohbayat@sbmu.ac.ir
- KMID: 2353591
- DOI: http://doi.org/10.5625/lar.2016.32.3.160
Abstract
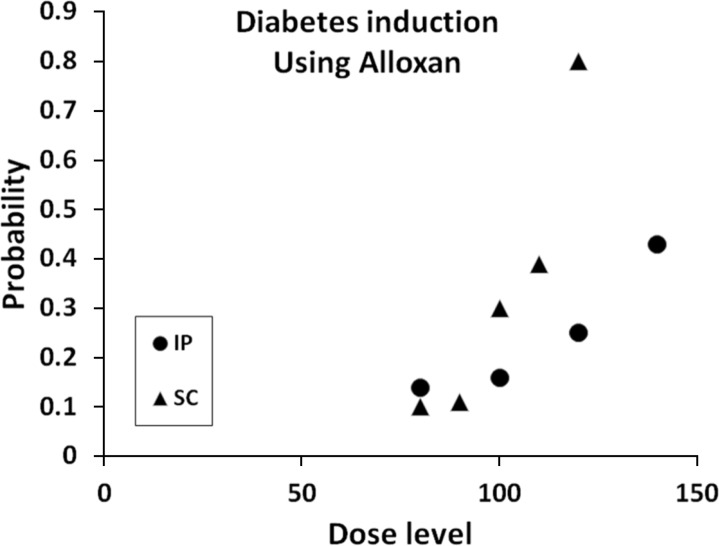
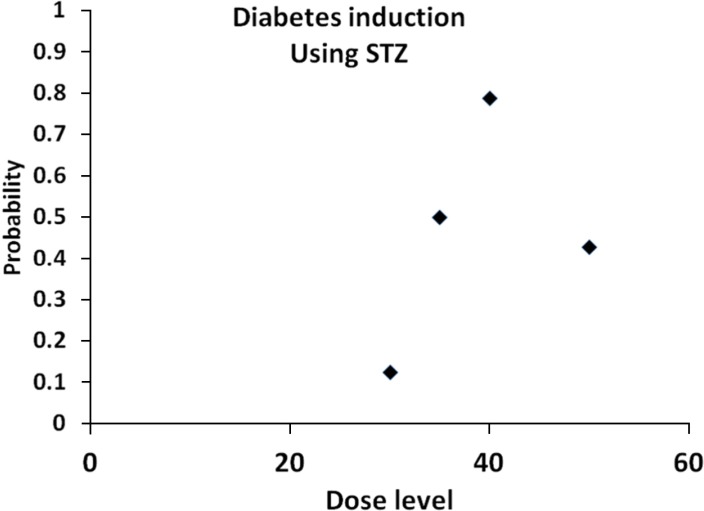
- The approach and novelty of this scientific work was to formulate the appropriate Streptozotocin (STZ) and Alloxan dosage in different routes of administration to imply minimum mortality rate and high incidence of diabetes mellitus (DM) in the rat experiment model. Rats were randomly divided into STZ, Alloxan and control groups. 1-Alloxan group was divided into two subgroups: intraperitoneal (ip) subgroups which received a single dose of, 140, 120, 100 and 80 mg/kg; and the subcutaneous (sc) subgroups which received a single dose of, 120, 110, 100, 90, and 80 mg/kg. 2-STZ group was divided into four subgroups of ip route. The ip subgroup which received intraperitoneally a single dose of, 30, 35, 40 and 50 mg/kg. 3-The control group: This group received solo distilled water. The injection day was considered as the day zero. Blood glucose levels and mortality rate were recorded. Subsequently, 30 days after, the logistic regression modeling was used to evaluate the effect of the explanatory variables, the dose levels, and route approaches, on the probability of DM incidence, and mortality. According to the statistical logistic analysis for Alloxan, it is concluded that the minimum dosage needed to induce DM was 120 mg/kg by sc method (probability 0.712). In addition, the logistic analysis for STZ showed that the optimal dose-level for STZ was 40 mg/kg with ip with approximate induction of DM probability 0.764. Based on the data, male Wistar rats in which received a single dosage of Alloxan by sc injection at dose of 120 mg/kg showed the most desirable result of induction of type I DM; furthermore, those in which received STZ by ip injection at the dose of 40 mg/kg developed a persistent and optimal DM state characterized by high rate of DM induction and low- level of mortality.
Keyword
MeSH Terms
Figure
Reference
-
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014; 37:S81. PMID: 24357215.2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010; 87(1):4–14. PMID: 19896746.
Article3. Agbaje IM, Rogers DA, McVicar CM, McClure N, Atkinson AB, Mallidis C, Lewis SE. Insulin dependant diabetes mellitus: implications for male reproductive function. Hum Reprod. 2007; 22(7):1871–1877. PMID: 17478459.
Article4. Etuk EU. Animal models for studying diabetes mellitus. Agric Biol JN Am. 2010; 1(2):130–134.5. Federiuk IF, Casey HM, Quinn MJ, Wood MD, Ward WK. Induction of type-1 diabetes mellitus in laboratory rats by use of alloxan: route of administration, pitfalls, and insulin treatment. Comp Med. 2004; 54(3):252–257. PMID: 15253270.6. Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am J Pathol. 1979; 95(3):663–673. PMID: 377994.7. Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper E. The use of animal models in the study of diabetes mellitus. In Vivo. 2009; 23(2):245–258. PMID: 19414410.8. Rees DA, Alcolado JC. Animal models of diabetes mellitus. Diabet Med. 2005; 22(4):359–370. PMID: 15787657.
Article9. King AJ. The use of animal models in diabetes research. Br J Pharmacol. 2012; 166(3):877–894. PMID: 22352879.
Article10. Hoftiezer V, Carpenter AM. Comparison of streptozotocin and alloxan-induced diabetes in the rat, including volumetric quantitation of the pancreatic islets. Diabetologia. 1973; 9(3):178–184. PMID: 4268550.
Article11. Gajdosik A, Gajdosikova A, Stefek M, Navarova J, Hozova R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999; 18:54–62. PMID: 10703720.12. Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian J Med Res. 2007; 125(3):451–472. PMID: 17496368.13. Ar'Rajab A, Ahren B. Long-term diabetogenic effect of streptozotocin in rats. Pancreas. 1993; 8(1):50–57. PMID: 8419909.14. Chougale AD, Panaskar SN, Gurao PM, Arvindekar AU. Optimization of Alloxan Dose is Essential to Induce Stable Diabetes for Prolonged Period. Asian J Biochem. 2007; 2(6):402–408.15. Jain DK, Arya RK. Anomalies in alloxan-induced diabetic model: It is better to standardize it first. Indian J Pharmacol. 2011; 43(1):91. PMID: 21455436.
Article16. Kalinichev M, Robbins MJ, Hartfield EM, Maycox PR, Moore SH, Savage KM, Austin NE, Jones DN. Comparison between intraperitoneal and subcutaneous phencyclidine administration in Sprague-Dawley rats: a locomotor activity and gene induction study. Prog Neuropsychopharmacol Biol Psychiatry. 2008; 32(2):414–422. PMID: 17945407.
Article17. Inoue Y, Kiryu S, Izawa K, Watanabe M, Tojo A, Ohtomo K. Comparison of subcutaneous and intraperitoneal injection of Dluciferin for in vivo bioluminescence imaging. Eur J Nucl Med Mol Imaging. 2009; 36(5):771–779. PMID: 19096841.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Diabetes Mellitus on the Disposition of Tofacitinib, a Janus Kinase Inhibitor, in Rats
- Experimental animal models for diabetes and its related complications—a review
- Effect of Melatonin on the Diabetes Mellitus Induced by Streptozotocin in Rats
- Antihyperglycemic and Antihyperlipidemic Effects of Fermented Rhynchosia nulubilis in Alloxan-induced Diabetic Rats
- Therapeutic Effect of Amomum xanthoides Extract on Experimental Diabetes Induced by Alloxan