Blood Res.
2016 Sep;51(3):171-174. 10.5045/br.2016.51.3.171.
Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy
- Affiliations
-
- 1Department of Laboratory Medicine, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital, Jinju, Korea. ehkohmd@gnu.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital, Jinju, Korea.
- 3Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- KMID: 2353497
- DOI: http://doi.org/10.5045/br.2016.51.3.171
Abstract
- BACKGROUND
Unfractionated heparin (UFH) has unstable pharmacokinetics and requires close monitoring. The activated partial thromboplastin time (aPTT) test has been used to monitor UFH therapy for decades in Korea, but its results can be affected by numerous variables. We established an aPTT heparin therapeutic range (HTR) corresponding to therapeutic anti-Xa levels for continuous intravenous UFH administration, and used appropriate monitoring to determine if an adequate dose of UFH was applied.
METHODS
A total of 134 ex vivo samples were obtained from 71 patients with a variety of thromboembolisms. All patients received intravenous UFH therapy and were enrolled from June to September 2015 at Gyeongsang National University Hospital. All laboratory protocols were in accordance with the Clinical and Laboratory Standards Institute guidelines and the College of American Pathologist requirements for aPTT HTR.
RESULTS
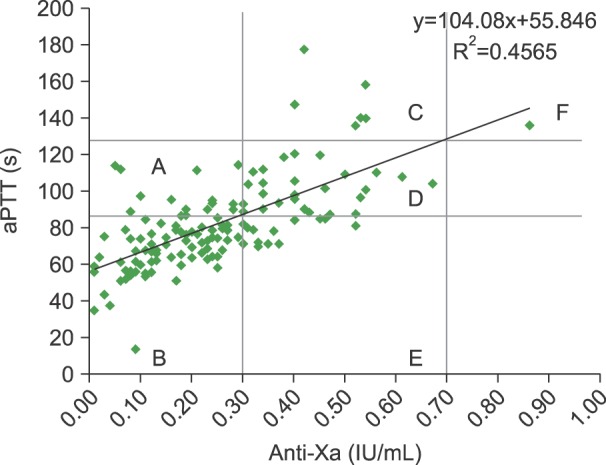
An aPTT range of 87.1 sec to 128.7 sec corresponded to anti-Xa levels of 0.3 IU/mL to 0.7 IU/mL for HTR under our laboratory conditions. Based on their anti-Xa levels, blood specimen distribution were as follows: less than 0.3 IU/mL, 65.7%; 0.3-0.7 IU/mL (therapeutic range), 33.6%; and more than 0.7 IU/mL, 0.7%. No evidence of recurring thromboembolism was observed.
CONCLUSION
Using the conventional aPTT target range may lead to inappropriate dosing of UFH. Transitioning from the aPTT test to the anti-Xa assay is required to avoid the laborious validation of the aPTT HTR test, even though the anti-Xa assay is more expensive.
Keyword
Figure
Reference
-
1. Guervil DJ, Rosenberg AF, Winterstein AG, Harris NS, Johns TE, Zumberg MS. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 2011; 45:861–868. PMID: 21712506.
Article2. Baglin T, Barrowcliffe TW, Cohen A, Greaves M. British Committee for Standards in Haematology. Guidelines on the use and monitoring of heparin. Br J Haematol. 2006; 133:19–34. PMID: 16512825.
Article3. Warkentin TE, Crowther MA. Reversing anticoagulants both old and new. Can J Anaesth. 2002; 49:S11–S25. PMID: 12557411.4. McGlasson DL, Kaczor DA, Krasuski RA, Campbell CL, Kostur MR, Adinaro JT. Effects of pre-analytical variables on the anti-activated factor X chromogenic assay when monitoring unfractionated heparin and low molecular weight heparin anticoagulation. Blood Coagul Fibrinolysis. 2005; 16:173–176. PMID: 15795534.
Article5. Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012; 32:546–558. PMID: 22531940.
Article6. Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972; 287:324–327. PMID: 5041701.
Article7. College of American Pathologist. College of American Pathologist hematology and coagulation laboratory accreditation checklist. Northfield, IL: College of American Pathologists;2007. February 3, 2016. at http://www.cap.org/web/home/lab/accreditation/accreditation-checklists.8. The Clinical and Laboratory Standards Institute (CLSI). One-stage prothrombin time (PT) test and activated partial thromboplastin time (APTT) test; Approved Guideline-Second edition. Wayne, PA: The Clinical and Laboratory Standards Institute;2008.9. Garcia DA, Baglin TP, Weitz JI, Samama MM. American College of Chest Physicians. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141(2 Suppl):e24S–e43S. PMID: 22315264.10. Cuker A, Ptashkin B, Konkle BA, et al. Interlaboratory agreement in the monitoring of unfractionated heparin using the anti-factor Xa-correlated activated partial thromboplastin time. J Thromb Haemost. 2009; 7:80–86. PMID: 19017257.11. Cuker A, Raby A, Moffat KA, Flynn G, Crowther MA. Interlaboratory variation in heparin monitoring: Lessons from the Quality Management Program of Ontario coagulation surveys. Thromb Haemost. 2010; 104:837–844. PMID: 20664895.
Article12. Lehman CM, Frank EL. Laboratory monitoring of heparin therapy: partial thromboplastin time or anti-Xa assay? Lab Med. 2009; 40:47–51.
Article13. Kostousov V, Nguyen K, Hundalani SG, Teruya J. The influence of free hemoglobin and bilirubin on heparin monitoring by activated partial thromboplastin time and anti-Xa assay. Arch Pathol Lab Med. 2014; 138:1503–1506. PMID: 25357112.
Article14. Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-xa measurements in heparin monitoring: biochemical basis for discordance. Am J Clin Pathol. 2013; 139:450–456. PMID: 23525615.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Establishing Therapeutic Ranges of Activated Partial Thromboplastin Time for Heparin Therapy using Anti-Xa Activity
- Should therapeutic range in heparin therapy be changed with new reagent for activated partial thromboplastin time?
- Enoxaparin-associated Retroperitoneal Hematoma in Two Chronic Dialysis Patients
- Monitoring of Heparin Treatment in Acute Cerebral Infarction
- Enhancing Safety of Heparin Administration: An Analysis of Prolonged aPTT and Bleeding in Patients Receiving Heparin