Lab Med Online.
2016 Oct;6(4):233-239. 10.3343/lmo.2016.6.4.233.
Performance Evaluation of Two Automated Quantitative Fecal Occult Blood Tests
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center and University of Ulsan College of Medicine, Seoul, Korea. mnkim@amc.seoul.kr
- KMID: 2353425
- DOI: http://doi.org/10.3343/lmo.2016.6.4.233
Abstract
- BACKGROUND
The performance of the fecal occult blood test (FOBT) has recently improved with the use of quantitative immunochemical assays. We evaluated the two latest immunochemical FOBTs: OC-Sensor PLEDIA (Eiken Chemical, Japan) and NS-Prime (Alfresa Pharma, Japan).
METHODS
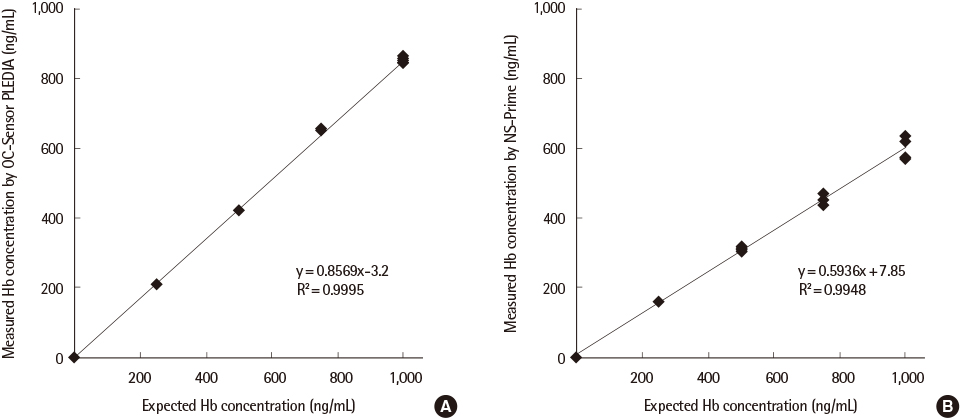
The precision was evaluated by using the quality control materials at two levels and carry-over rates were measured at high and low concentrations of the sample, prepared from the calibrators. Linearity was measured by using five concentrations of human hemoglobin (0-1,000 ng/mL), prepared from erythrocyte lysates. Correlation between the two systems was analyzed by testing approximately 50 selected stool specimens per day and comparing the results obtained with those of the currently used analyzer, OC-Sensor DIANA (Eiken Chemical), for 10 consecutive working days.
RESULTS
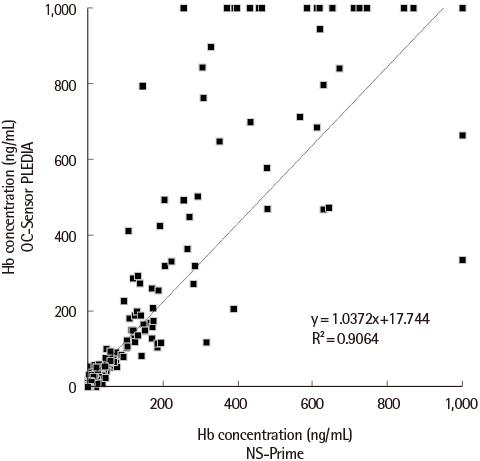
The variation for repeatability, between-run, between-day, and intermediate precision at both levels was <5.0%, and carry-over rates were <0.01% for both systems. Linearity slopes were 0.857 and 0.594 for PLEDIA and NS-Prime, respectively, with r²>0.99 for both systems. In total, 499 stool specimens were analyzed, of which 127 (25.5%), 130 (26.1%), and 129 (25.9%) specimens tested positive by DIANA, PLEDIA, and NS-Prime, respectively. The agreement between PLEDIA and NS-Prime was 98.4%. Quantification by PLEDIA was linear to that by NS-Prime (y=1.0372x+17.744; r²=0.9064).
CONCLUSIONS
The analytical performances of PLEDIA and NS-Prime warrant their use as diagnostic tests. They showed excellent categorical agreement; however, the quantitative value obtained by NS-Prime was lower than that obtained by PLEDIA.
Figure
Cited by 1 articles
-
Performance Evaluation of SENTiFIT 270 and FOB Gold Reagent for Detecting Fecal Occult Blood
Da Young Kang, Dokyun Kim, Keonhan Kim, In-Ho Jang, Seok Hoon Jeong
Ann Clin Microbiol. 2019;22(2):29-34. doi: 10.5145/ACM.2019.22.2.29.
Reference
-
1. von Karsa L, Patnick J, Segnan N, Atkin W, Halloran S, Lansdorp-Vogelaar I, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis: overview and introduction to the full supplement publication. Endoscopy. 2013; 45:51–59.
Article2. Kwon JH, Choi MG, Suh JP, Chang JH, Nam KW, Park HS, et al. The significance of fecal occult blood testing to screen for colon cancer. Korean J Gastrointest Endosc. 2007; 35:68–73.3. Lim JU, Bae NY, Song WK, Cha JM, Lee JI. The significance of Fecal Immunochemical Test to Screen for Colorectal Cancer in National Cancer Screening Program. Intest Res. 2010; 8:126–134.
Article4. Lamph S, Bennitt W, Brannon C, Halloran S. Evaluation report: immunochemical faecal occult blood tests. United Kingdom: National Health Service: Purchasing and Supply Agency;2009.5. Lee YK. 진단검사의 표준화와 건강보험 재정영향 분석연구. http://www.prism.go.kr/homepage/researchCommon/retrieveResearchDetailPopup.do?research_id=1351000-201500177 2014.6. Jeon CH, Lee AJ, Kim KD. Annual report on external quality assessment scheme for urinalysis and faecal occult blood testing in Korea (2014). J Lab Med Qual Assur. 2015; 37:179–189.
Article7. Allison JE. Colon Cancer Screening Guidelines 2005: the fecal occult blood test option has become a better FIT. Gastroenterology. 2005; 129:745–748.
Article8. Allison JE, Tekawa IS, Ransom LJ, Adrain AL. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996; 334:155–159.
Article9. Kim JH, Chung HJ, Yoon NS, Pyo YJ, Bae HG, Kim MN. Evaluation of the OC-SENSOR neo system for testing fecal occult blood. Korean J Lab Med. 2007; 27:210–215.
Article10. Horiuchi Y, Masubuchi J, Oikawa S, Matsuda R, Hishinuma A, Ieiri T. Evaluation of fully automated fecal occult blood analyzer OC-SENSOR neo and OC-SENSOR II. Jap Assoc Clin Lab Auto. 2003; 28:40–46.11. Okuyama Y, Doi Y, Matsuyama N, Uchino M, Yamamoto T. A novel sol particle immunoassay for fecal calprotectin in inflammatory bowel disease patients. Clin Chim Acta. 2016; 456:1–6.
Article12. Tholen DW, Kallner A, Kennedy JW, Krouwer JS, Meier K. Evaluation of precision performance of quantitative measurement methods; approved guideline—second edition. CLSI document EP5-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2004.13. McEnroe R, Durham A, Goldford M, Kondratovich M, Lababidi S, Magari R, et al. Evaluation of precision of quantitative measurement procedures: approved guideline. CLSI document EP5-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2014.14. Daniel W, Martin K, Astles J. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI document EP6-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2003.15. Krouwer JS, Cembrowski GS, Tholen DW. Preliminary evaluation of quantitative clinical laboratory measurement procedures; approved guideline. CLSI document EP10-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2006.16. Budd J, Durham A, Gwise T, Iriarte B, Kallner A, Linnet K, et al. Measurement procedure comparison and bias estimation using subject samples, approved quideline. CLSI document EP09-A3. Wayne, PA: Clinical and Laboratory Standards Institute;2013.17. Hong SB, Kim HS, Park HS, Lee DH. Evaluation of the HM-JACK automatic analyzer for fecal occult blood test. J Lab Med Qual Assur. 2002; 24:221–224.18. Kim DC, Cho SS, Song J, Kim EC, Kim JQ. Evaluation of the OC-SENSOR (Automatic Measuring Apparatus) for immunological fecal occult blood test. J Clin Pathol Qual Control. 1998; 20:281–287.19. González AG, Herrador MÁ. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends in Analytical Chemistry. 2007; 26:227–238.
Article20. Schiettecatte J, Anckaert E, Smitz J. Interferences in immunoassays. Adv Immunoass Technol. 2012.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the HM-JACKarc Analyser for Fecal Occult Blood Test
- Evaluation of Hemo Techt NS-plus C15 Automatic Analyzer for Fecal Occult Blood Test
- Evaluation of the OC-SENSOR neo System for Testing Fecal Occult Blood
- Evaluation of the OC-SENSOR (Automatic Measuring Apparatus) for Immunological Fecal Occult Blood Test
- Evaluation of the Ez step FOBTM for Fecal Occult Blood Test