Lab Med Online.
2016 Oct;6(4):228-232. 10.3343/lmo.2016.6.4.228.
Changing Guidelines for Clinical Microbiology Laboratories and Their Influences on Workflows Related to Consultations
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. deyong@yuhs.ac
- KMID: 2353424
- DOI: http://doi.org/10.3343/lmo.2016.6.4.228
Abstract
- BACKGROUND
Since the concept of 'minimal identification of poor quality specimens or microbes with low pathogen potential' has been introduced into the standard operating procedure (SOP) to enhance work efficiency, consultations are requested for further species identification and antimicrobial susceptibility testing. The aim of this study was to evaluate the impact of consultations requests to the clinical microbiology laboratory on its work efficiency.
METHODS
From January 2013 to April 2015, consultation requests to the laboratory in a tertiary-care hospital were collected from electronic medical records. The characteristics of consultations and changes to workflow due to the laboratory SOP amendment were analyzed. Turnaround time of the consultation and specimen culture were evaluated as an indicator of workflow efficiency.
RESULTS
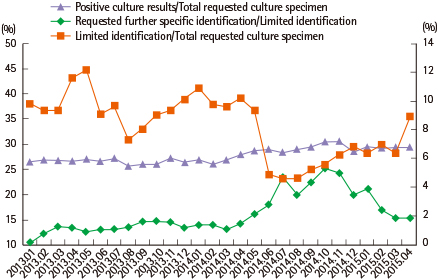
A total of 971 consultations were evaluated during the study period. The most common purposes for consultations were microbe species identification and antimicrobial susceptibility tests. Among the minimal identification reports, the proportions of consultations were below 5%. The number of consultations had increased substantially. However, the turnaround time of consultation and specimen culture showed declining trends.
CONCLUSIONS
With the introduction of the consultation system, the workload for species identification and antimicrobial susceptibility testing of colonizing microbes could be minimized. This research provides an example of work efficiency management for laboratory procedures based on an SOP amendment.
Figure
Reference
-
1. Abbott M, Paulin H, Sidhu D, Naugler C. Laboratory tests, interpretation, and use of resources: a program to introduce the basics. Can Fam Physician. 2014; 60:e167–e172.2. Benson ES. The responsible use of the clinical laboratory. Clin Biochem. 1986; 19:262–270.
Article3. Garcia , et al. Clinical microbiology procedures handbook. 3rd ed. Washington DC: ASM Press;2010.4. Lyu SM, Byun JY, Choi YW, Choi HY. Clinical features of dermatology-consulted inpatients - focus on the differences between individual departments. Korean J Dermatol. 2014; 52:215–221.5. Lee HY, Go SE, Son SH, Kim MK, Lee GH, Hyun MS. Inpatient consultations by the hematology department over a 1-year period. Korean J Med. 2009; 76:578–583.6. Clinical and Laboratory Standards Institute. Performances standards for antimicrobial susceptibility testing. Twenty-fiftht Informational supplement, M100-S25. Wayne, PA: Clinical and Laboratory Standards Institute;2015.7. Kim TS, Lee K, Hong YJ, Hwang SM, Park JS, Park KU, et al. MALDI-TOF MS: its application in the clinical laboratory and a paradigm shift in clinical microbiology. Lab Med Online. 2015; 5:176–187.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laboratory diagnosis of Clostridioides difficile infection: guidelines and status of practice in Korea
- Nationwide Survey of Blood Culture Protocol in Clinical Microbiology Laboratories in Korea
- Challenging diagnosis of parasitic infection and practical guidance to clinical microbiology laboratories in Korea
- Biosafety of Microbiological Laboratories in Korea
- Fungal infections: rising threats, diagnostic challenges, and the path forward for clinical microbiology laboratories